Table of Contents
Opening statement:
Plasmon-induced chemical transformations have become a fascinating subject of study in the discipline of chemistry, primarily because of their ability to initiate and expedite chemical processes in normal environmental circumstances. These reactions use the distinctive characteristics of plasmons, which are the collective vibrations of electrons on the surface of metal nanoparticles. This makes plasmons an effective instrument for facilitating chemical processes that are otherwise difficult to accomplish. Because they are important in both biological and synthetic chemistry, secondary amides stand out among the many substrates that can go through plasmon-driven changes.
Surface-Enhanced Raman Scattering (SERS) is a groundbreaking technology that has transformed how we investigate chemical interactions on a molecular scale. The Raman signal of molecules attached to uneven metal surfaces is greatly improved by SERS. This makes the method very sensitive and specific. This makes it perfect for investigating chemical changes mediated by plasmons.
Analyzing Plasmon-Driven Chemical Reactions:
To comprehend the importance of plasmon-driven chemical processes, it is crucial to have a clear understanding of the nature and mechanisms of plasmons. Plasmons are the collective movements of electrons that occur on the surface of metallic nanoparticles, specifically noble metals like gold or silver. Upon exposure to light, the nanoparticles transfer energy to the plasmons, resulting in the formation of a strongly confined electromagnetic field. Plasmon-driven chemistry is a phenomenon in which strong fields stimulate and accelerate chemical reactions on nanoparticle surfaces. To
The plasmon-driven reactions occur because of the localized electromagnetic fields’ ability to reduce activation energy barriers and enhance bond-breaking and bond-forming activities. These catalysts are very valuable for promoting reactions that are otherwise sluggish or necessitate severe conditions, hence creating opportunities for advancements in synthetic chemistry.
The structure and importance of secondary amides are of particular interest:
This type of amide has a nitrogen atom connected to two alkyl or aryl groups and a carbonyl group. It is important in many chemical and biological processes. They are found in peptides, proteins, and several synthetic polymers, making them essential components of both biological systems and industrial uses.
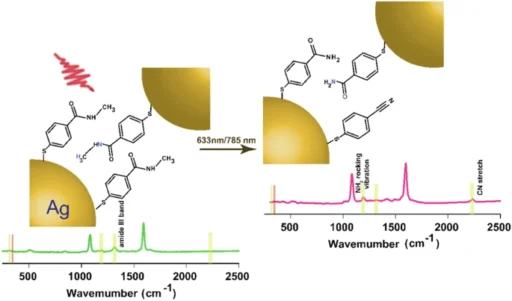
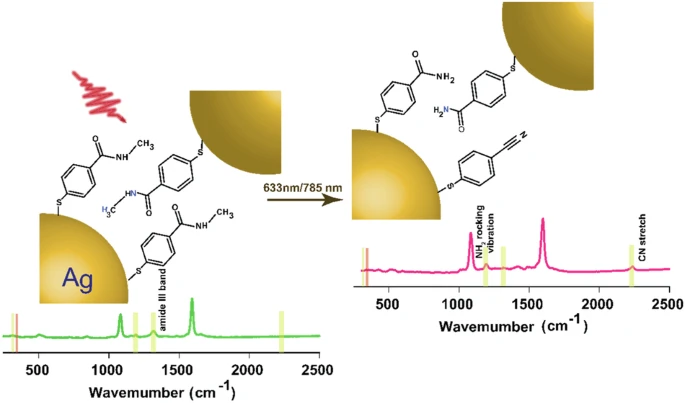
Secondary amides pose a challenge in their study because they are relatively unreactive, and it is difficult to modify their chemical structure without altering their function. This makes them a compelling choice for plasmon-induced transformations, where the goal is to achieve specific alterations under gentle conditions. Overall time evolution of the transformation of a secondary amide tracked by SERS.
Surface-enhanced Raman Scattering (SERS) is an influential analytical technique:
Molecules scatter light in a way that alters its energy, a process known as Raman scattering. By analyzing the unique molecular fingerprint this phenomenon produces, we can identify and investigate different chemical species. Nevertheless, the limited intensity of the Raman signal frequently restricts its usefulness in identifying analytes with low concentrations. Surface-Enhanced Raman Scattering (SERS) is relevant in this context.
SERS significantly amplifies the Raman signal, enabling the detection and analysis of individual molecules in ideal circumstances. The localized surface plasmon resonance (LSPR) of metal nanoparticles, which enhances the electromagnetic field at the surface and increases the Raman signal of adsorbed molecules, is primarily responsible for the improvement.
Probing Chemical Transformations with SERS:
SERS is very suitable for investigating chemical transformations due to its ability to offer real-time, in situ monitoring of reactions at the molecular level. Through the examination of the alterations in the Raman spectrum during a reaction, scientists can gain valuable knowledge about the reaction mechanism, detect intermediate species, and ascertain the final products with great precision.
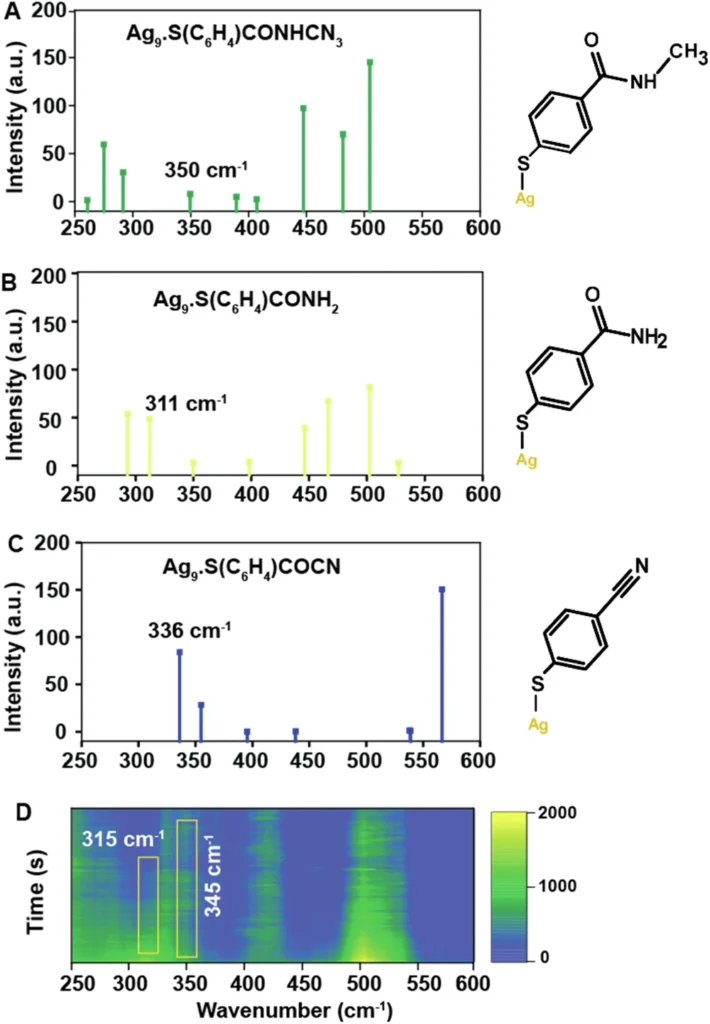
SERS is particularly useful for investigating plasmon-induced chemical reactions because it allows direct monitoring of the plasmonic field’s impact on the reaction. Such an outcome can facilitate a deeper understanding of the fundamental mechanisms and the development of more effective catalytic systems. Comparison of calculated Raman spectra and experimental SERS spectra.
Plasmon-Driven Chemical Transformation of Secondary Amides: Understanding the Mechanism
By activating the carbonyl group through the confined plasmonic field, plasmon-driven procedures commonly convert secondary amides. This activation enables the occurrence of nucleophilic attacks or other reaction pathways that would otherwise necessitate more severe circumstances.
In an experimental setup, metal nanoparticles, typically gold or silver, absorb a secondary amide onto their surface. A certain wavelength of light stimulates the plasmons in these nanoparticles, creating a strong electromagnetic field. This field interacts with the amide, reducing the amount of energy needed to initiate the transformation and allowing the reaction to occur under mild conditions.
Methods of experimentation:
The methodology for investigating plasmon-induced chemical conversions of secondary amides consists of several critical stages:
Preparation of Metal Nanoparticles: The process of creating metal nanoparticles involves synthesizing and analyzing gold or silver nanoparticles to determine their plasmonic properties.
Adsorption of Secondary Amides: Adsorption is the process of attaching the desired secondary amide to the surface of nanoparticles.
SERS Measurement: The sample undergoes Raman spectroscopy, utilizing the nanoparticles to enhance the SERS effect.
Reaction Monitoring: Reaction monitoring entails initiating the reaction by illuminating the sample with a certain wavelength of light, and then tracking the reaction’s progress by observing changes in the Raman spectrum.
Results and analysis:
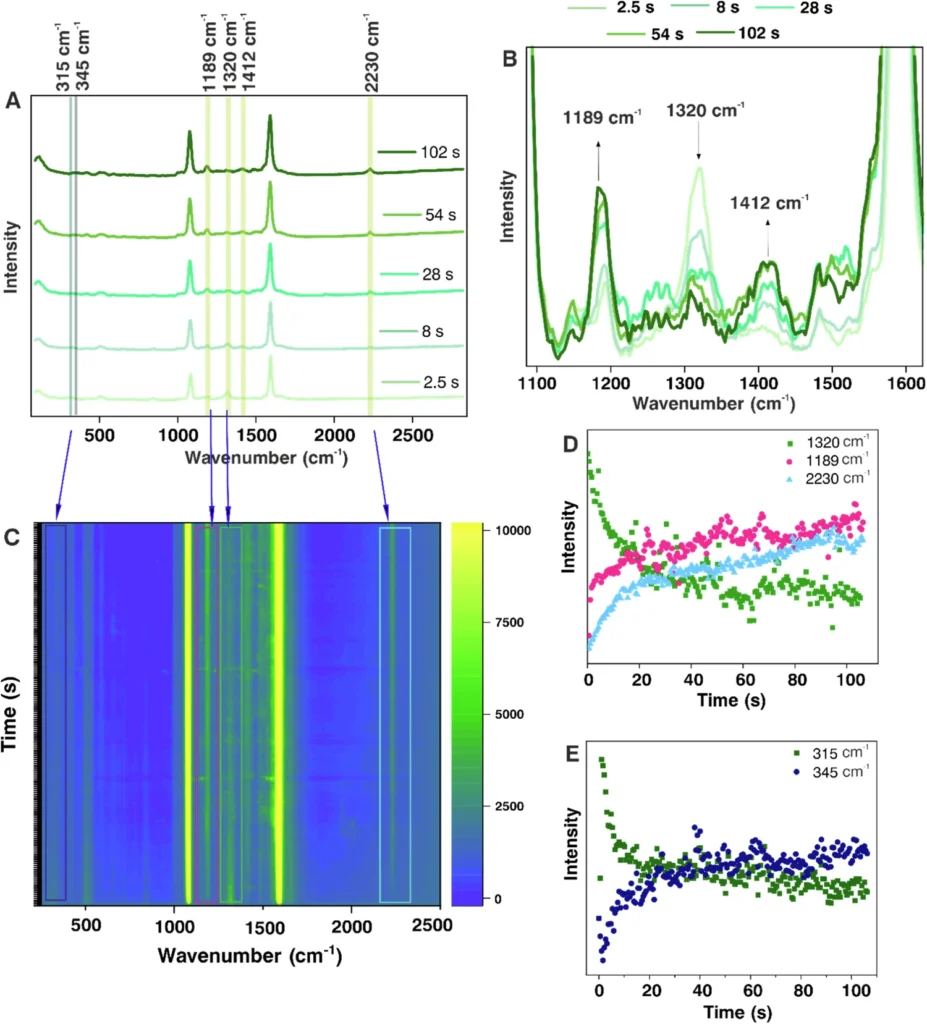
As the reaction driven by plasmons advances, the SERS data obtained from the experiment demonstrates notable alterations in the Raman spectra of the secondary amide. Peaks corresponding to the carbonyl stretch and other functional groups experience shifts or variations in intensity, which indicate the presence of a chemical transition.
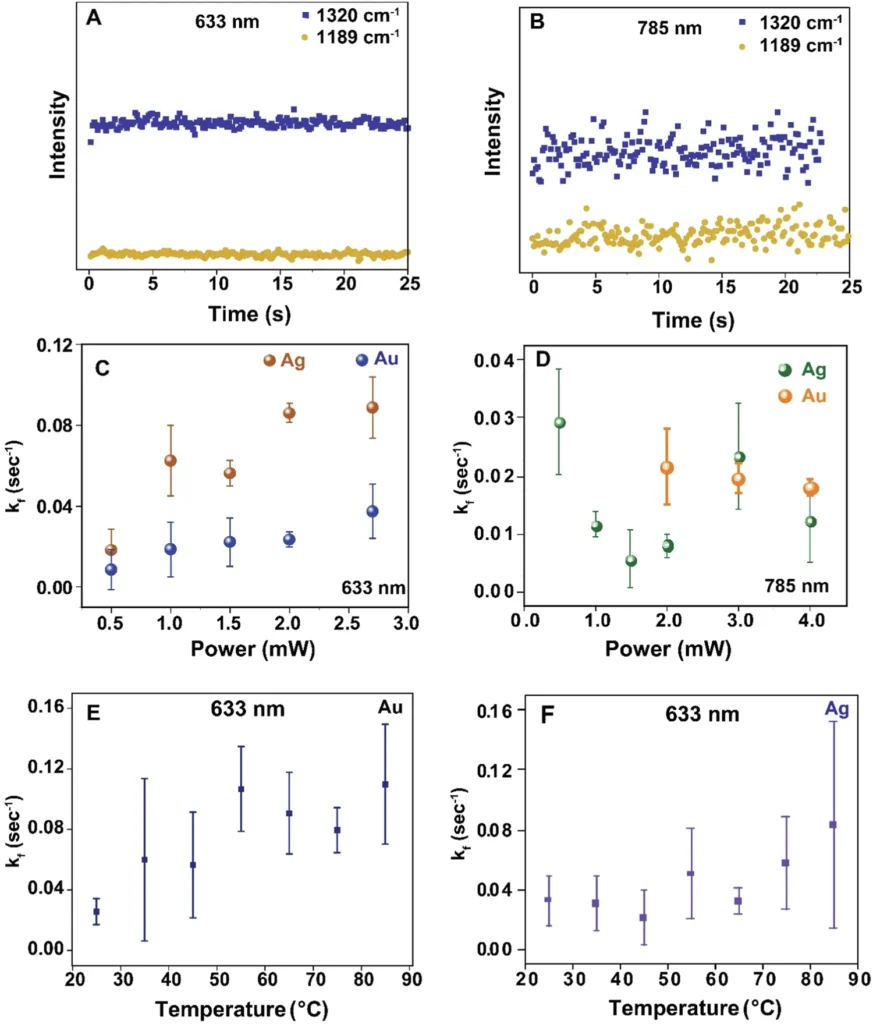
We can analyze the observed alterations in the spectrum to gain a deeper understanding of the reaction process. For example, a drop in the strength of the carbonyl stretch could mean that there was a nucleophilic attack on the carbonyl carbon, which led to the formation of a new product. Reactivity of NMSB molecule without nanoparticle and reaction rate study of the NMSB molecule with nanoparticle under different laser power and temperature conditions.
Difficulties in Investigating Plasmon-Induced Reactions via Surface-Enhanced Raman Spectroscopy (SERS):
Although SERS is a highly effective instrument, it can present several difficulties. Changes in the nanoparticle surface or the arrangement of the adsorbed molecules can influence the ability to reproduce SERS signals, a major concern. In addition, the analysis of SERS data can be intricate, necessitating meticulous examination to prevent misidentification of spectral characteristics.
To overcome these obstacles, it is necessary to employ a careful and precise experimental design. Additionally, in certain situations, it may be necessary to create novel nanoparticle substrates that offer more reliable enhancement and improved regulation of the reaction environment.
Utilization of Plasmon-Driven Chemical Transformations:
Inducing chemical reactions with Plasmons has a wide range of practical applications. Plasmon-driven transformations have potential applications in the pharmaceutical industry, such as the selective modification of medicinal molecules or the precise synthesis of complicated chemicals. These reactions can also benefit environmental chemistry by breaking down contaminants or transforming waste materials into valuable products.
We anticipate a closer integration of Plasmon-driven chemistry with other technologies like microfluidics and artificial intelligence to improve the efficiency and scalability of chemical processes.
Comparative analysis with alternative analytical methodologies:
Although SERS provides notable benefits in terms of sensitivity and specificity, it is not the sole tool accessible for investigating chemical changes. Infrared spectroscopy (IR), nuclear magnetic resonance (NMR), and mass spectrometry (MS) are distinct techniques with individual advantages and drawbacks.
IR spectroscopy is highly effective in analyzing functional groups; however, it is not as sensitive as SERS. Nuclear Magnetic Resonance (NMR) offers intricate insights into molecule structure; however, it necessitates substantial sample quantities. Mass spectrometry allows for an accurate assessment of molecular weight, but it does not directly reveal information about the chemical surroundings.
Prospects for Advancements in Surface-Enhanced Raman Spectroscopy (SERS) and Chemistry Driven by Plasmonics:
We expect the area of Surface-Enhanced Raman Spectroscopy (SERS) to continue its rapid progress in the future. We expect the accuracy and dependability of SERS measurements to improve with the progress in nanoparticle production and the development of novel plasmonic materials. Furthermore, the integration of SERS with other analytical techniques has the potential to generate novel hybrid methodologies that use the advantages of multiple methods.
Future plasmon-driven chemistry research will strive to expand the range of reactions plasmons can facilitate, while simultaneously improving the selectivity and efficiency of these processes. This has the potential to result in substantial progress in fields such as catalysis, materials science, and green chemistry.
Environmental and industrial consequences:
The plasmon-driven chemical reactions have significant promise for environmental advantages. By facilitating reactions to take place at low temperatures and pressures, these techniques have the potential to decrease the energy usage and waste generated by conventional chemical methods. This is consistent with the objectives of green chemistry, which aim to reduce the ecological consequences of chemical manufacturing.
The adoption of plasmon-driven processes in industry will heavily depend on their scalability. While current research primarily focuses on small-scale studies, the application of plasmon-driven chemistry concepts in large-scale manufacturing processes could lead to more environmentally friendly and efficient production methods.
In conclusion:
Investigating plasmon-induced chemical changes in secondary amides using surface-enhanced Raman scattering provides a unique opportunity to observe the complex dynamics of molecular-level chemical processes. This method not only improves our comprehension of the underlying principles but also expands the potential for utilizing plasmon-driven reactions in other areas of chemistry.
Further advancements in this field are likely to lead to inventive applications of plasmon-driven chemistry, particularly in the development of environmentally friendly and sustainable chemical processes. Together with the ongoing progress in nanoparticle technology and the addition of surface-enhanced Raman spectroscopy (SERS) to other analytical methods, this strong approach will become much more useful.
Frequently Asked Questions:
1). What are plasmons and why are they important in chemical reactions?
They play a crucial role in chemical reactions due to their ability to enhance the interaction between light and matter, leading to more efficient energy transfer and catalytic processes. Plasmons are the coordinated vibrations of electrons on the surface of metal nanoparticles. They play a crucial role in chemical reactions as they generate powerful electromagnetic fields, which can facilitate chemical transformations by reducing the barriers of activation energy.
2). What are the differences between SERS and conventional Raman spectroscopy?
SERS significantly boosts the Raman signal of molecules absorbed on metal surfaces, leading to a significantly higher sensitivity in comparison to conventional Raman spectroscopy. This improvement enables the identification of individual molecules, even in specific circumstances.
3). What makes secondary amides significant in chemistry?
Secondary amides are of great importance in chemistry due to their notable properties and versatile applications. Secondary amides have a crucial role in various biological compounds, including proteins and peptides, and are also significant in synthetic chemistry. Because of their generally unreactive nature and resistance to alteration, they pose a challenge, yet they are highly important targets for chemical transformation.
4). What are the challenges associated with using Surface-Enhanced Raman Spectroscopy (SERS) to investigate chemical reactions?
Challenges include the ability to replicate SERS signals, the possibility of interference from other adsorbed species, and the complexity of interpreting SERS spectra. To tackle these problems, it is necessary to employ meticulous experimental design and sophisticated data processing tools.
5). What is the role of plasmon-driven chemical reactions in promoting green chemistry?
Plasmon-induced reactions can take place at low temperatures and pressures, minimizing the requirement for strong reagents and substantial energy inputs. This enhances their environmental friendliness and is under the principles of green chemistry.
For more chemistry blogs, visit chemistry Master