Table of Contents
Preface:
Plasmonic photocatalysis is a novel technology situated at the convergence of nanotechnology, chemistry, and physics. It utilizes the distinctive characteristics of plasmonic materials—commonly noble metals such as gold and silver—that can absorb and alter light at the nanoscale. Exposure of these materials to light induces the excitation of plasmons, which are collective oscillations of electrons. This excitation creates new avenues for facilitating chemical reactions, many of which are essential for energy conversion, environmental remediation, and industrial operations.
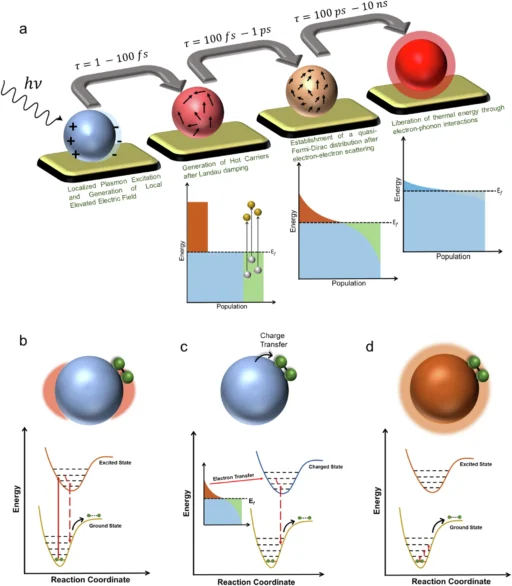
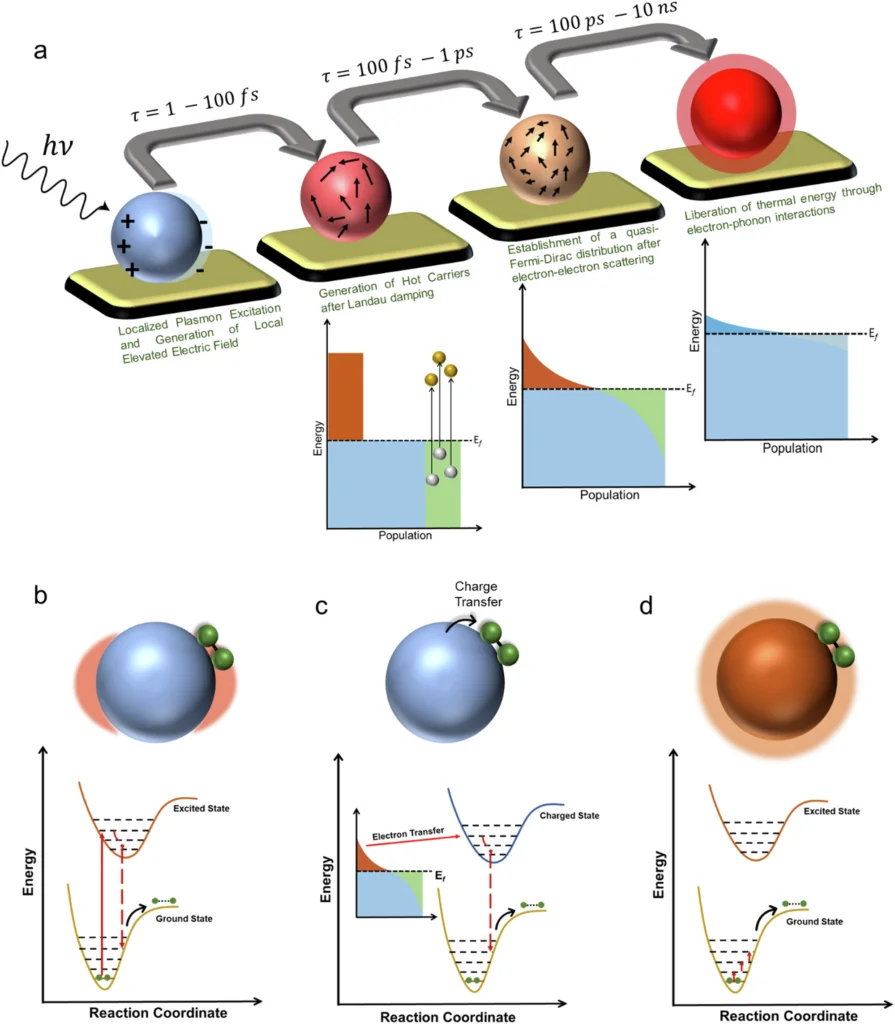
A significant contradiction has arisen in the field: the challenge of differentiating between thermal and non-thermal effects in plasmonic photocatalysis. Both effects transpire concurrently; however, they possess markedly distinct mechanisms and ramifications for the efficiency and specificity of catalytic operations. Understanding this contradiction is essential for enhancing plasmonic photocatalysis in practical applications. What are the thermal and non-thermal effects? What makes their separation so challenging? What are the ramifications of resolving this paradox? Schematic representation of LSPR-induced processes and effects.
What is plasmonic photocatalysis?
Plasmonic photocatalysis refers to the use of plasmonic nanoparticles to facilitate chemical processes following exposure to light irradiation. In contrast to conventional catalysis, which predominantly utilizes heat to drive reactions, plasmonic photocatalysis depends on light to initiate or enhance processes. This light-driven catalysis is attractive as it provides a more sustainable alternative to conventional procedures, which generally necessitate elevated temperatures and substantial energy inputs.
The historical discovery of plasmonics began in the early 20th century; however, it wasn’t until the late 1990s and early 2000s that plasmonic photocatalysis began to emerge as a significant area of research. The ability to manipulate light at the nanoscale and focus it into specific spatial regions, facilitated by plasmon resonance, has made it an invaluable tool in chemical reactions. Researchers are currently investigating plasmonic photocatalysis in domains such as hydrogen generation, carbon dioxide reduction, water dissociation, and pollution remediation.
Comprehending Plasmons: The Core of Plasmonic Photocatalysis
Plasmons are fundamental to plasmonic photocatalysis. Collective oscillations of free electrons arise when light interacts with a metallic surface, usually at the nanoscale. These oscillations produce a robust electromagnetic field adjacent to the metal surface, condensing light into a significantly smaller volume than its initial wavelength would indicate. This energy concentration can subsequently facilitate chemical reactions in adjacent molecules.
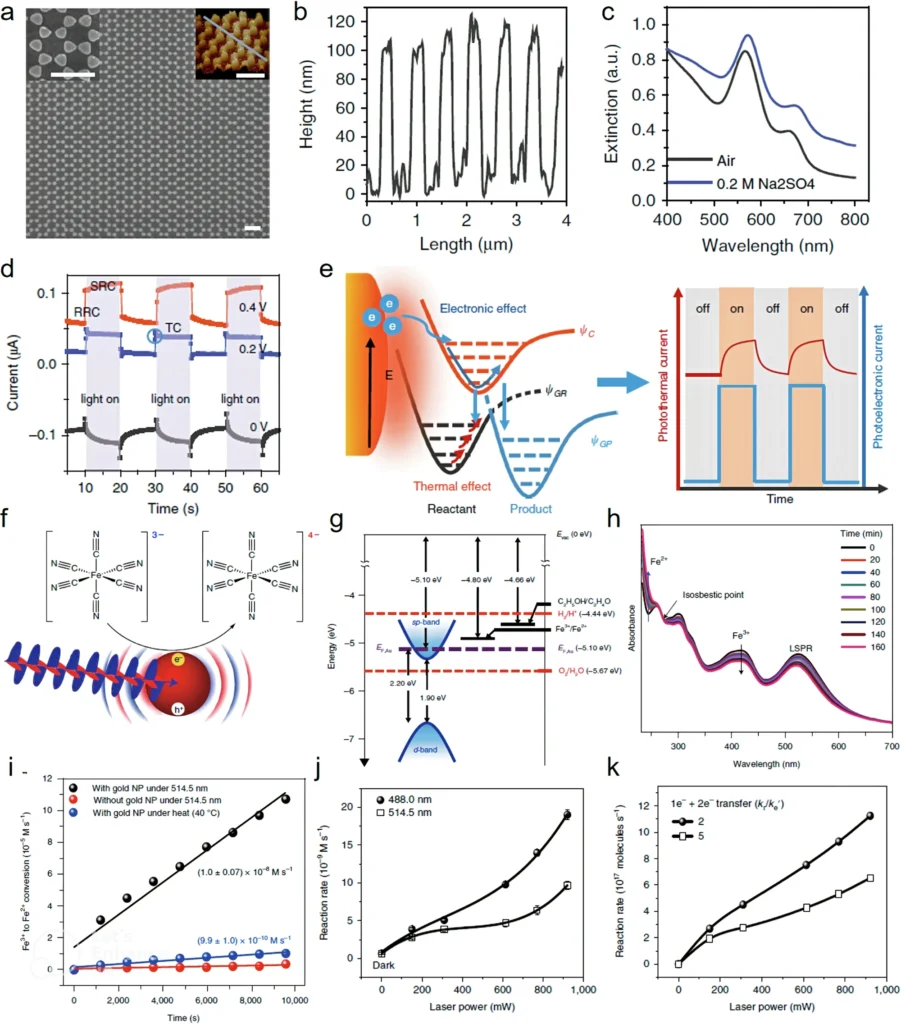
When light hits a plasmonic material, two primary processes occur: the material may generate heat, resulting in thermal effects, or it may yield highly energetic electrons and holes, resulting in non-thermal effects. The simultaneous occurrence of these processes makes it difficult to identify the primary driver of a certain reaction. This is the inception of the paradox. Slow and fast response of Au nanoelectrode array and Fe3+ reduction by Au nanoparticle.
Thermal Impacts in Plasmonic Photocatalysis:
Thermal effects in plasmonic photocatalysis refer to the heat produced during plasmon decay. This heat can significantly raise the local temperature of the catalyst and its environment, thereby accelerating the reaction rate. This is a known occurrence in conventional catalysis, where elevating the system’s temperature typically accelerates the reaction, as articulated by the Arrhenius equation.
In plasmonic photocatalysis, the plasmonic material internally generates heat instead of externally applying it. When plasmons decay, they emit energy as phonons (vibrational modes of the atomic lattice), increasing the material’s temperature. Powered by light instead of external heat sources, this localized heating can enhance reaction rates similar to conventional thermal catalysis, but it allows for better control precision.
In oxidation reactions, such as the oxidation of organic compounds on gold nanoparticles, heat effects frequently prevail. As the temperature of the nanoparticle rises owing to plasmonic heating, the reaction rate increases. Researchers have often noticed that the rate of the reaction changes with temperature, which is typical for processes that are driven by heat. As the temperature rises, the chances of reactant molecules passing the activation energy barrier rise.
Non-thermal phenomena in plasmonic photocatalysis:
Non-thermal effects, on the other hand, result from direct energy transfer from excited plasmons to reactant molecules without a significant temperature rise. One of the most significant non-thermal mechanisms is the formation of hot carriers, which are highly energetic electrons and holes produced during the decay of plasmons. Adjacent molecules can introduce these energetic carriers, which directly transfer their energy to the reactants, triggering chemical reactions.
This method can facilitate reactions that would typically require significantly elevated temperatures to occur. For instance, plasmon excitation can transfer heated electrons to CO2 molecules during the reduction process, thereby promoting their transformation into valuable compounds like carbon monoxide or methane. In contrast to thermal effects, which typically hasten reactions indiscriminately, non-thermal effects can offer enhanced control over the reaction route, resulting in more selective and energy-efficient processes.
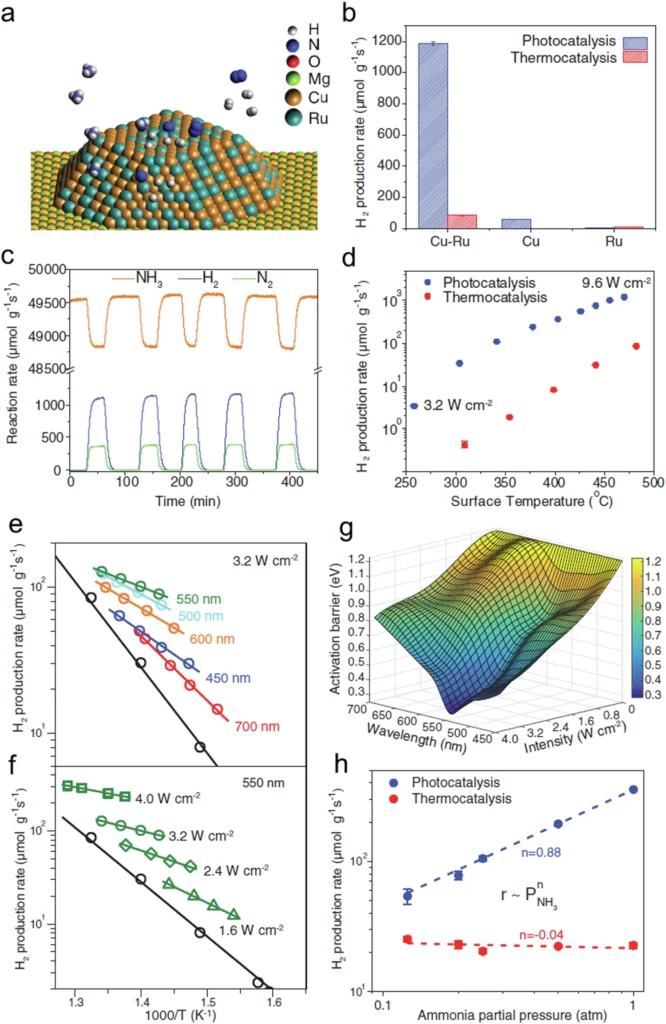
Non-thermal factors significantly influence reactions like water splitting, where heated electrons can facilitate the production of hydrogen and oxygen from water. In such cases, the ability to exploit non-thermal effects facilitates reactions at lower temperatures, reducing the required energy input and improving the process’s sustainability. Comparative analysis of photocatalytic and thermocatalytic performance of Cu-Ru-AR.
The Paradox: The Intricacy of Thermal vs. Non-Thermal Effects
The dilemma of thermal and non-thermal effects emerges from their simultaneous occurrence in plasmonic photocatalysis. When a plasmonic nanoparticle is irradiated with light, it produces both thermal effects (heat) and non-thermal effects (hot carriers). In some instances, it is challenging to ascertain which of these factors predominantly accounts for the observed catalytic reaction. This ambiguity hampers the design of catalytic systems, as some applications may derive greater advantages from one effect over the other.
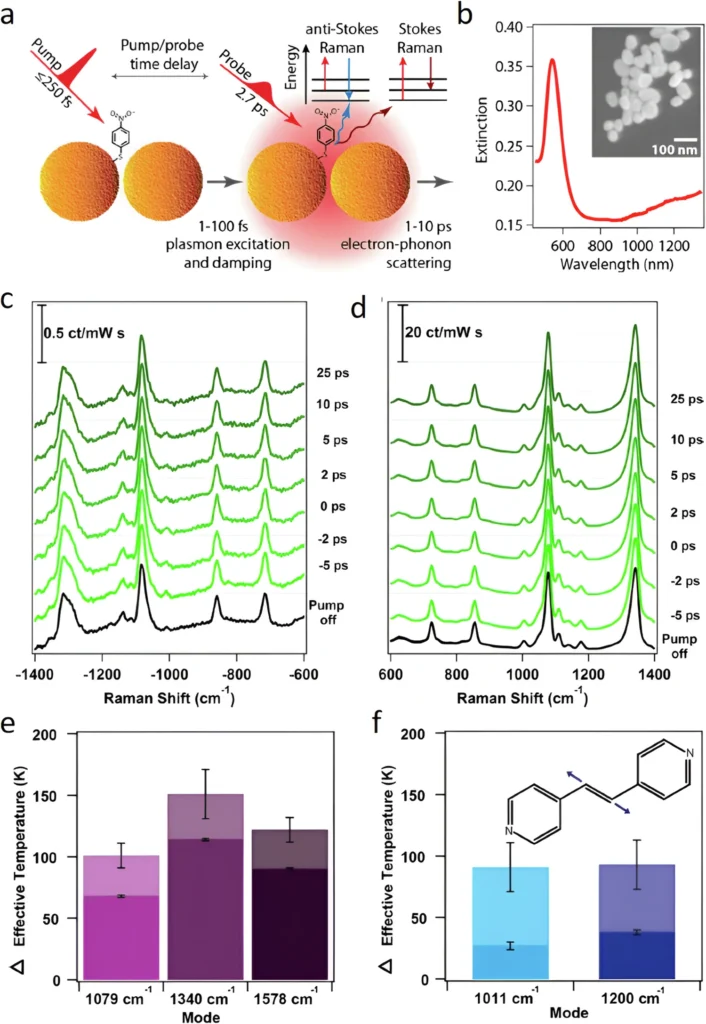
The differentiation is critical, as thermal and non-thermal effects have varying consequences for catalytic efficiency and reaction selectivity. If thermal effects dominate a reaction, increasing the light intensity will only raise the temperature, which could lead to faster but less selective reactions. If non-thermal factors predominate, the reaction may occur with greater selectivity and at reduced temperatures, thereby resulting in more efficient and controlled operations. Ultrafast Raman thermometry and temperature dynamics in plasmonic photocatalysts.
Differentiating Thermal from Non-Thermal Effects: Challenges
A significant obstacle in addressing this dilemma is the difficulty in experimentally differentiating between thermal and non-thermal effects. In many instances, the heat produced by plasmon decay can raise the surrounding temperature by tens or even hundreds of degrees Celsius, complicating the exclusion of thermal effects even when non-thermal mechanisms are assumed.
Furthermore, the factors that impact plasmonic photocatalysis—namely particle size, morphology, and light wavelength—can influence both thermal and non-thermal mechanisms. Smaller nanoparticles typically generate a greater number of hot carriers, but larger particles are likely to produce increased heat. The complexity implies that minor alterations in experimental settings can alter the equilibrium between thermal and non-thermal effects, complicating the isolation of one process from the other.
Experimental Methods for Distinguishing Thermal from Non-Thermal Effects:
To tackle these issues, researchers have devised many experimental methodologies to distinguish between thermal and non-thermal effects in plasmonic photocatalysis. Temperature-controlled tests, which meticulously manage heat settings to see the reaction, are a prevalent method. By externally regulating the system’s temperature, researchers can evaluate whether the reaction rate accelerates due to plasmonic heating or non-thermal phenomena such as hot electron transfer.
Researchers have used ultrafast transient absorption spectroscopy and other spectroscopic techniques to monitor the real-time behavior of hot electrons. These techniques enable researchers to examine the kinetics of electron excitation and decay, yielding significant insights into non-thermal phenomena. Through the comparison of electron behavior across many experimental configurations, researchers can start to elucidate the influences of thermal and non-thermal phenomena.
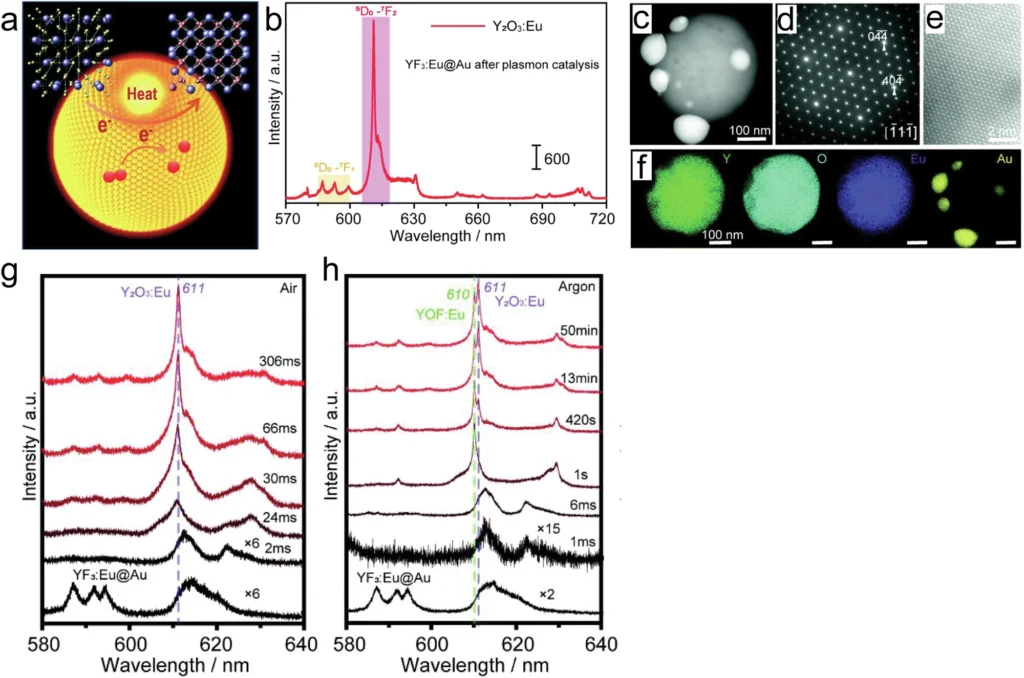
Computational simulations serve as robust instruments for examining the dilemma. By simulating the behavior of plasmons and their interactions with reactant molecules, researchers can forecast the manifestation of various effects under differing situations. These simulations can inform experimental design and enhance comprehension of the underlying mechanisms involved. Non-thermal and thermal effects in crystal transformation from YF3 to Y2O3.
Thermal Effects: An in-depth examination
The same principles that regulate conventional thermal catalysis frequently elucidate thermal effects in plasmonic photocatalysis. As the temperature of a catalyst rises, the molecules involved in the reaction acquire kinetic energy, allowing them to surmount the activation energy barrier with greater ease. Reaction rates generally escalate with temperature, as articulated by the Arrhenius equation.
Plasmon decay produces thermal energy in plasmonic systems that can elevate the local temperature of the catalyst and its environment, thereby accelerating reaction rates. This targeted heating is particularly beneficial in reactions that require elevated temperatures for optimal efficiency, such as methane reforming or hydrocarbon oxidation. In these situations, we can use the thermal effects of plasmonic materials to achieve the necessary reaction conditions without the need for external heating.
Non-Thermal Effects: Mechanistic Insights
Non-thermal effects depend on the direct interaction between light-excited plasmons and reactant molecules. When a plasmon decays, it produces hot carriers, which are highly energetic electrons and holes, which can spread to nearby molecules and initiate chemical reactions. This method fundamentally differs from thermal catalysis, as it does not depend on elevating the system’s temperature.
A primary advantage of non-thermal effects is their ability to facilitate reactions at lower temperatures than thermal effects alone would permit. Plasmon excitation can introduce hot electrons into CO2 molecules during the reduction process, thereby facilitating their transformation into valuable compounds like carbon monoxide or methane. This process can transpire at ambient temperature, rendering it a more energy-efficient substitute for conventional thermal catalysis.
Non-thermal influences can enhance the regulation of reaction pathways and product selectivity. Because of their elevated energy levels, hot carriers can initiate certain processes that would not occur under standard thermal conditions. This selectivity is crucial in intricate reactions, as managing the reaction pathway can result in increased yields of the intended product.
Interaction Between Thermal and Non-Thermal Effects:
Thermal and non-thermal effects often coexist and collaborate, facilitating catalytic processes. Plasmonic materials produce thermal energy, which can enhance the efficiency of hot electron transfer by increasing the energy of reactant molecules, thereby enabling them to accept hot electrons. Non-thermal factors can also reduce a process’s activation energy, allowing for a more rapid progression at higher temperatures.
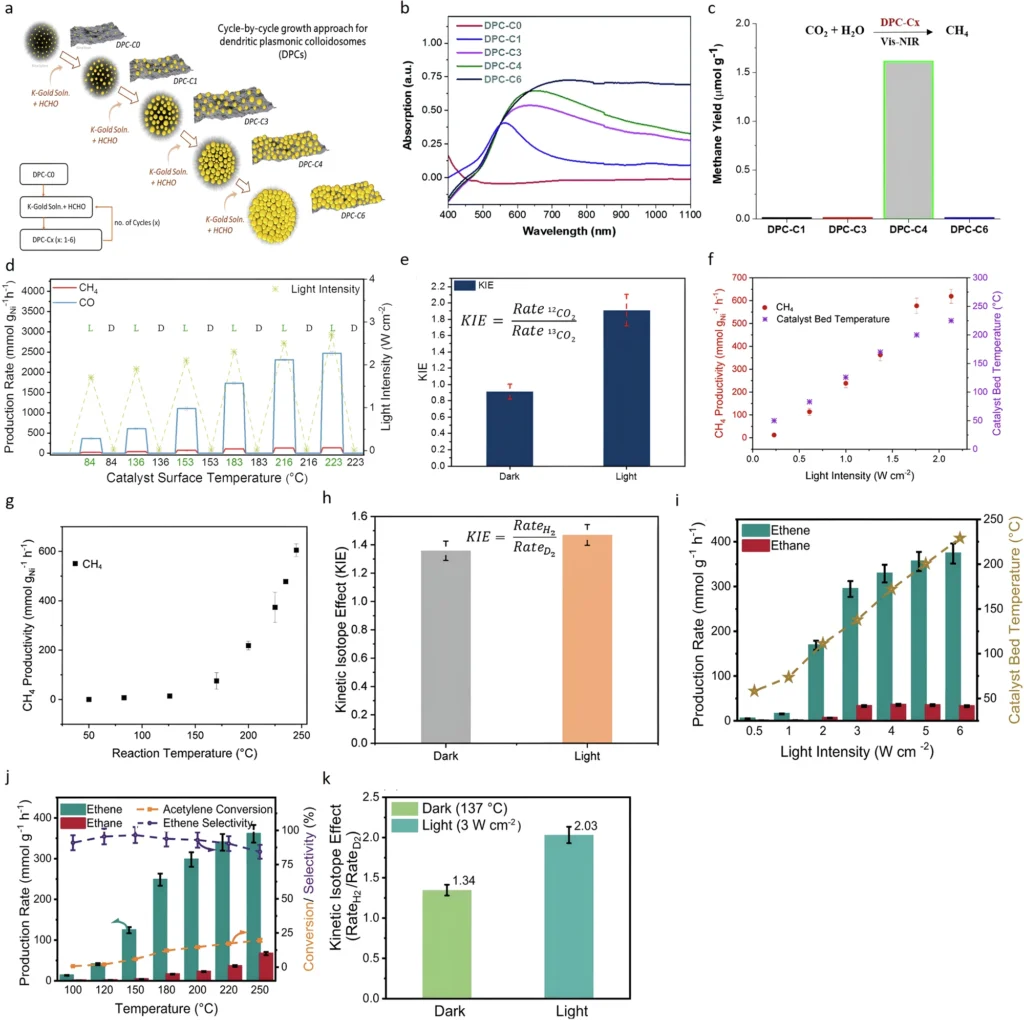
The interaction between thermal and non-thermal effects is a key factor contributing to the efficacy of plasmonic photocatalysis. By leveraging both effects, researchers can develop catalytic systems that are more efficient and adaptable than those dependent solely on thermal or non-thermal phenomena. Nonetheless, understanding the interaction of these effects in specific reactions remains a significant problem, necessitating additional research to fully realize their potential. Thermal and non-thermal pathways in black gold assisted plasmonic reactions.
The influence of material characteristics on the equilibrium of thermal and non-thermal effects is significant:
The characteristics of the plasmonic material significantly determine the equilibrium between thermal and nonthermal effects in plasmonic photocatalysis. Particle size, shape, and content can influence the relative impacts of thermal and non-thermal processes.
Smaller nanoparticles typically produce a greater number of heat carriers, hence amplifying non-thermal effects. Larger particles are more effective at producing heat, leading to a heightened impact of thermal effects. The material’s composition is significant, as various metals exhibit distinct plasmonic characteristics. While gold is known for its ability to create hot carriers, silver excels in heat generation.
The external environment, including the intensity and wavelength of incident light, can affect the equilibrium between thermal and non-thermal effects. Increased light intensities often elevate heat production; however, shorter light wavelengths can generate more energetic hot carriers. By meticulously regulating these factors, researchers may optimize the catalytic properties of plasmonic materials to preferentially enhance one action over another.
Pragmatic Consequences for Catalytic Efficacy:
To make plasmonic photocatalysis work better, it is important to understand and control the balance between thermal and non-thermal effects. In certain instances, optimizing thermal effects may be advantageous, particularly in reactions that necessitate elevated temperatures for effective progression. In certain instances, non-thermal effects may prove more advantageous, especially in processes where selectivity and energy efficiency are paramount.
By customizing the characteristics of the plasmonic material and the reaction parameters, researchers can engineer catalytic systems that optimize both effects. In solar-to-chemical energy conversion processes, non-thermal effects can facilitate reactions at lower temperatures, reducing the required energy input. Thermal effects can simultaneously accelerate reaction rates, thereby boosting the system’s overall efficiency.
Theoretical Frameworks to Elucidate the Paradox:
Scholars have proposed numerous theoretical models to clarify the conflict between thermal and non-thermal effects in plasmonic photocatalysis. These models aim to elucidate the conditions under which each effect prevails and their interactions in specific reactions.
A popular model, based on classical electrodynamics, explains how light interacts with plasmonic materials and generates heat as a result. This model can forecast the heat produced by plasmon decay and its impact on response rates. Nonetheless, it fails to comprehensively address the non-thermal consequences of hot carriers, necessitating a quantum mechanical framework.
To understand the behavior of hot carriers in plasmonic systems, scientists have created quantum mechanical models. These models take into account the energy distribution of hot electrons and holes, as well as their interactions with reactant molecules. Researchers are increasingly developing complete models that elucidate the duality of thermal and non-thermal effects by integrating classical and quantum methodologies. Plasmonic nanostructures for enhanced hydrogen evolution reaction in aqueous solutions and kinetic switch.
Emerging Trends in Research: Addressing the Paradox
As plasmonic photocatalysis advances, researchers are formulating novel techniques to address the contradiction of thermal and non-thermal effects. A promising strategy involves the creation of plasmonic materials tailored to preferentially enhance one effect over another. By manipulating the dimensions and morphology of nanoparticles, researchers can fabricate materials that provide an increased number of hot carriers or elevated thermal energy, contingent upon the specific application required.
The use of sophisticated experimental methodologies to accurately regulate and quantify the impacts of plasmonic excitation is a burgeoning trend. Ultrafast spectroscopy enables researchers to monitor the behavior of heated carriers in real time, yielding significant insights into non-thermal processes. Temperature-controlled studies can effectively separate thermal effects, making it easier to examine their contributions to catalytic reactions.
Wider Implications for Energy and Environmental Technologies:
Addressing the dilemma of thermal versus non-thermal effects in plasmonic photocatalysis could transform various industries, especially in renewable energy and environmental sustainability. By utilizing non-thermal phenomena, researchers could create more effective methods for converting solar energy into chemical fuels, including hydrogen or methane. These techniques could substantially diminish our dependence on fossil fuels and assist in alleviating climate change.
Environmental applications may employ plasmonic photocatalysis to decompose contaminants or transform hazardous chemicals into less dangerous compounds. By comprehending the optimization of the equilibrium between thermal and non-thermal effects, researchers could devise catalytic systems that are more efficient, selective, and environmentally sustainable.
Final Assessment:
The dichotomy between thermal and nonthermal effects in plasmonic photocatalysis represents a particularly fascinating and complex challenge within the discipline. Despite significant progress in understanding the mechanisms of these effects, we still need to gain substantial knowledge about their interactions and how to regulate them for improved catalytic effectiveness.
We expect researchers investigating this contradiction to develop innovative materials, methodologies, and models that will push the boundaries of plasmonic photocatalysis. Resolving this conundrum would enable the full utilization of plasmonic materials to facilitate chemical reactions, resulting in more efficient and sustainable technologies across energy, industry, and environmental sectors.
Frequently Asked Questions:
1). In plasmonic photocatalysis, what is the primary distinction between thermal and non-thermal effects?
Thermal effects refer to heat generation from plasmonic excitation, whereas non-thermal effects are caused by the direct energy transfer from hot electrons and holes to reactant molecules.
2). How do researchers attempt to isolate these effects in experiments?
Researchers employ temperature-regulated experiments, sophisticated spectroscopy, and computational models to differentiate between thermal and non-thermal effects.
3). Can thermal and non-thermal influences synergistically enhance catalytic efficiency?
Indeed, thermal and non-thermal effects can sometimes synergize, resulting in more efficient and adaptable catalytic systems.
4). What are the practical applications of non-thermal effects in plasmonic catalysis?
Non-thermal effects are especially advantageous in processes such as CO2 reduction and water splitting, where hot electrons facilitate reactions at reduced temperatures, enhancing energy efficiency and selectivity.
5). In what manner could the resolution of this contradiction facilitate progress in renewable energy?
By comprehending and regulating the equilibrium between thermal and non-thermal effects, researchers could create more efficient methodologies for converting solar energy into chemical fuels, thus diminishing our dependence on fossil fuels and promoting renewable energy technology.
For more chemistry blogs, visit chemistry Master