Table of Contents
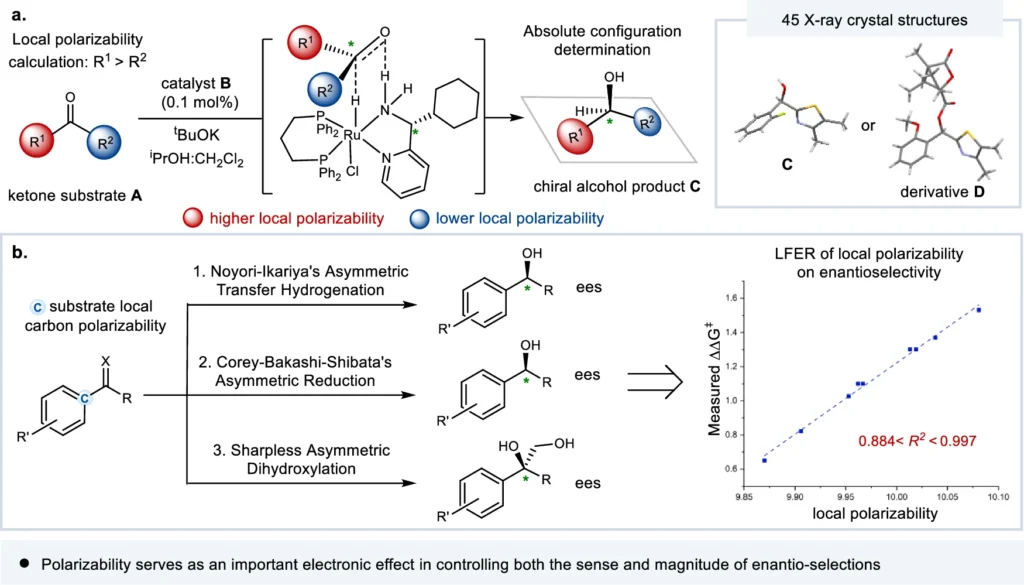
In enantio-selective processes, polarizability—a molecule’s ability to change the shape of its electron cloud when exposed to an outside electric field—is very important. Enantioselectivity is the process of making one enantiomer more likely to be made than the other. It is essential in organic chemistry, especially in medicine, because the effectiveness of chiral compounds depends on their specific stereochemistry.
An Overview of Polarizability and Enantioselectivity:
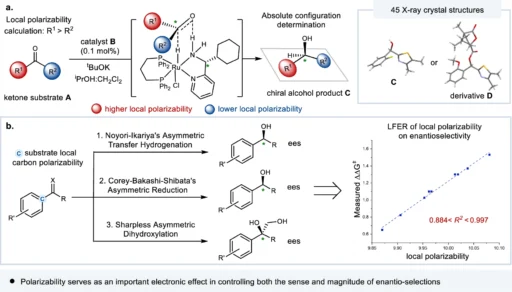
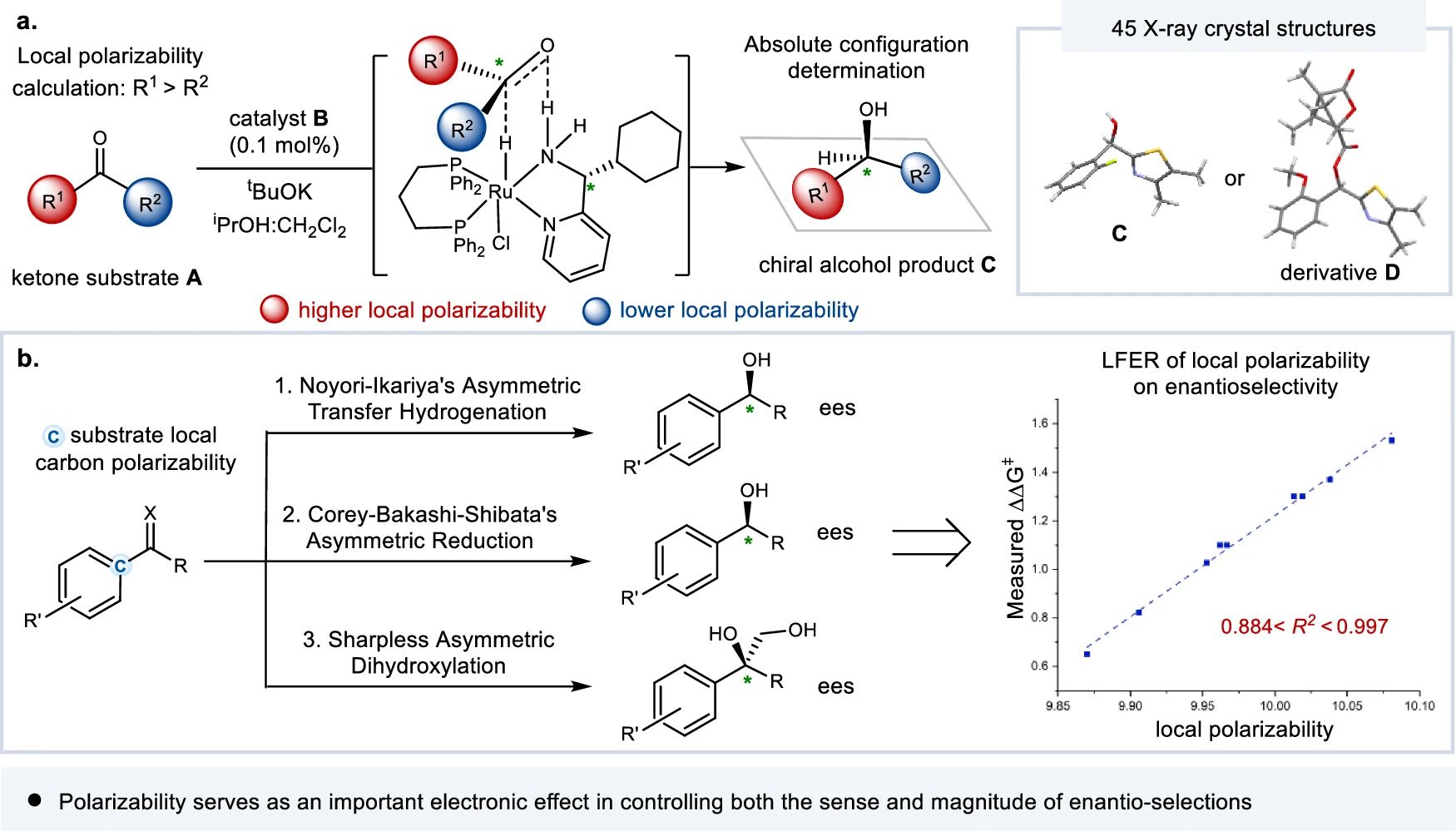
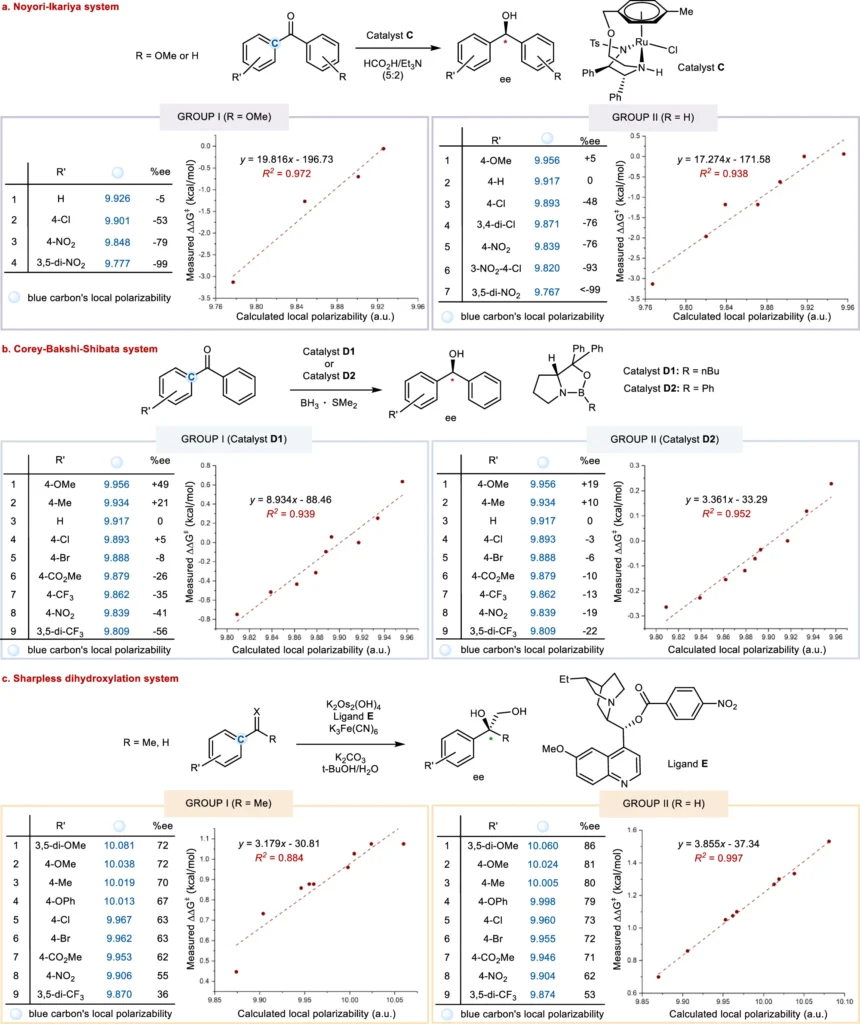
It is an intrinsic property of molecules that determines their susceptibility to changes in their electron distribution. It plays a crucial role in enantio-selective processes, which aim to create a specific enantiomer. It affects the interactions between chiral catalysts or reagents and the molecules involved in the reaction, eventually determining the stereochemical result. The essential role of local electronic polarizal in enantio-control (this work).
Comprehending Enantio-selectivity:
Enantiomers are each other’s non-superimposable mirror reflections. Enantio-selectivity is the capacity of a catalyst or reagent to preferentially produce one enantiomer over the other in a chiral environment. This characteristic is of utmost importance in creating organic compounds, as it directly influences the amount and quality of chiral products. These products, which possess asymmetry, play a vital role in the field of therapeutic research and advancement.
The significance of polarizability in enantioselective reactions:
Because it affects how chiral catalysts or reagents interact with enantiomers, it can make one enantiomer more likely to be made than the other. Asymmetric synthesis exploits the varying polarizability of enantiomers to selectively attach to chiral environments, thereby promoting the creation of the desired stereoisomer.
Variables Influencing Polarizability:
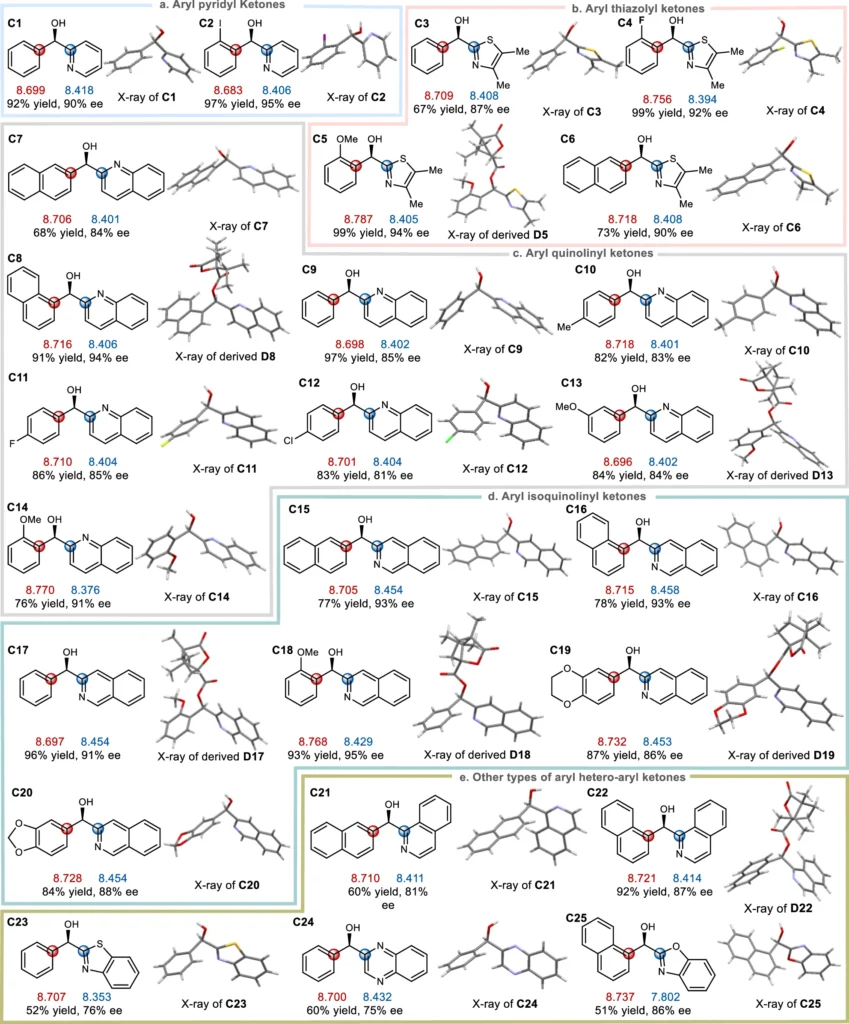
Various factors, such as a molecule’s chemical structure and the presence of substituents, influence its polarizability. Higher electron density functional groups tend to be more polarized, whereas groups that drain electrons from the molecule decrease its polarizability. Furthermore, the polarity of a solvent can influence a molecule’s polarizability, which in turn affects its reactivity in enantio-selective processes. Correlation of substrate substituent local electronic polarizability on the sense of asymmetric induction in Ru-catalyzed asymmetric transfer hydrogenation of aryl hetero-aryl ketones.
Methods for Evaluating Polarizability via Experimental Techniques:
Commonly employed approaches for evaluating the polarizability of molecules include nuclear magnetic resonance (NMR) spectroscopy and computational methods. NMR spectroscopy provides important insights into molecules’ electronic structure and dynamics, while computational approaches such as density functional theory (DFT) computations provide information on molecular characteristics and reactivity.
Utilization of Polarizability in Organic Synthesis:
Understanding it has led to the creation of catalytic systems and chiral ligands that use the different polarizability of enantiomers to make chemicals asymmetrically. To get high levels of enantio-control and efficiency in catalytic enantio-selective processes like asymmetric hydrogenation and asymmetric allylation, you have to precisely control it.
Recent developments in the polarizability research field:
The main focus of recent research has been on figuring out what it means in enantio-selective processes and coming up with new ways to measure and control it. The progress in spectroscopic techniques and computer modeling has yielded a significant understanding of the mechanisms involved in asymmetric catalysis, hence creating opportunities for the development of catalysts that are both more efficient and selective. Linear free energy relationship (LFER) analysis of substrate local polarizabilities on enantio-selections.
Obstacles and Prospects for the Future:
Although there has been notable advancement, there are still obstacles to comprehensively grasping and utilizing polarizability’s potential in enantio-selective processes. Researchers are actively developing new experimental tools and theoretical frameworks to analyze it, and designing unique catalysts and ligands. These areas show promise for overcoming present limits and furthering discipline.
In conclusion:
To sum up, it is an important part of enantio-selective processes that affect the stereochemical outcome of asymmetric synthesis. Chemists can enhance the efficiency and selectivity of catalytic systems for synthesizing chiral chemicals, which have various uses in fields like medicine and materials research, by comprehending and managing the polarizability of molecules.
Distinct Frequently Asked Questions:
1). What is polarizability’s role in determining enantioselectivity?
The ability to polarize is a key part of how chiral catalysts or reagents interact with enantiomers. It determines which enantiomer is produced over the other in asymmetric synthesis.
2). What are the experimental methods used to assess polarizability?
We apply commonly used techniques like NMR spectroscopy and computational approaches like density functional theory (DFT) computations to evaluate the polarizability of molecules and obtain information about their reactivity.
3). What are the implications or effects of polarizability in pharmaceutical research?
Understanding the significance of polarizability is vital to the development of effective catalytic systems for producing chiral pharmaceutical intermediates. Enantio-selectivity, which is instrumental in determining biological activity, is particularly important in this context.
4). Are there any constraints or restrictions on the existing techniques used for evaluating polarizability?
Although NMR spectroscopy and computer modeling offer useful insights into polarizability, there are still difficulties in precisely predicting and managing polarizability in complicated chemical systems.
5). What strategies may researchers employ to address the difficulties in utilizing polarizability for asymmetric synthesis?
Continued progress in developing new experimental techniques, computational approaches, and catalyst designs is crucial for surpassing existing constraints and propelling the field of enantio-selective catalysis forward.
For more chemistry blogs, visit chemistry Master