Table of Contents
Overview of propyltriethoxysilane:
Water pollution is a substantial worldwide problem that has a detrimental impact on the health and welfare of millions of individuals. Heavy metals, such as lead (II), pose a significant and hazardous risk when contaminating water sources. Industrial emissions, decaying infrastructure, and incorrect waste management frequently cause Lead(II) contamination, which can lead to significant health repercussions, particularly for children. Due to the high toxicity of lead (II) and its long-lasting presence in the environment, it is imperative to discover effective and efficient techniques for eliminating it from water.
In recent years, water remediation has made notable progress, particularly by creating innovative materials that can effectively absorb and eliminate pollutants. Graphene oxide (GO) is an auspicious material that, when modified with appropriate chemical groups, may efficiently target and eliminate lead (II) ions from polluted water. This study looks into how IDAPTES-GO, which is graphene oxide modified with (3-iminodiacetic acid) propyltriethoxysilane, can be used as a high-tech way to clean up water that has lead in it (II).
Analyzing Lead (II) Contamination:
Human activities primarily cause Lead(II) contamination in water. Typical sources of lead contamination include lead pipes and solder, industrial activities, mining, and the incorrect disposal of goods containing lead. When lead (II) enters the water system, it can remain there for a long time, which can cause serious health problems. These include cognitive impairment and developmental delays in children, renal damage, and cardiovascular concerns in adults.
Because of these potential dangers, regulatory authorities have implemented stringent regulations regarding the levels of lead (II) in drinking water. In many countries, the maximum permissible level of lead (II) in drinking water is 10 parts per billion (ppb). Nevertheless, even at such small levels, lead (II) might still pose a threat, emphasizing the urgency for efficient means of elimination.
Existing Techniques for Lead (II) Removal by using propyltriethoxysilane:
Conventional techniques for eliminating lead (II) from water consist of chemical precipitation, ion exchange, membrane filtration, and adsorption. Although these strategies can yield positive results, they frequently have inherent constraints. Chemical precipitation produces sludge that requires additional treatment; ion exchange can be expensive; and membrane filtration is susceptible to fouling.
Adsorption, employing substances such as activated carbon, is a highly prevalent technique renowned for its straightforwardness and effectiveness. Conventional adsorbents may exhibit restricted capacity and selectivity for lead(II) ions, especially when present in low quantities. These constraints emphasize the necessity for sophisticated materials capable of surpassing the disadvantages of current technologies and delivering enhanced cleanup of lead (II) in water.
Propyltriethoxysilane Graphene oxide is a highly promising material:
Propyltriethoxysilane Graphene oxide (GO) is a highly promising material in the realm of water treatment because of its distinct features. Propyltriethoxysilane Graphene oxide (GO) is a type of material that is two-dimensional and has a high surface area. Additionally, it possesses robust mechanical properties and readily adapts to various chemical groups. The properties of GO make it ideal for adsorbing heavy metals such as lead II.
GO has a distinct edge over typical adsorbents due to its ability to adjust and control its surface chemistry. By adding specific functional groups onto its surface, researchers can greatly enhance the adsorption capability and selectivity of GO for certain pollutants. The ability to customize GO-based materials enables researchers to create them according to specific purposes, such as the elimination of lead (II) from water.
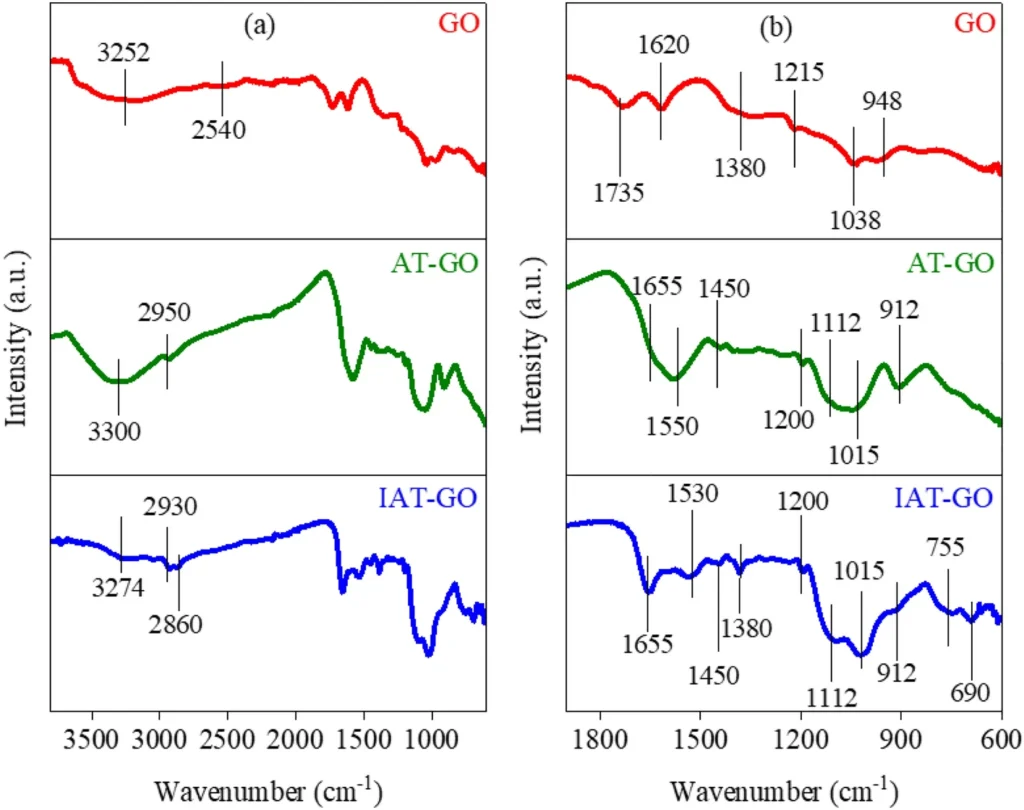
Propyltriethoxysilane Graphene oxide can be functionalized:
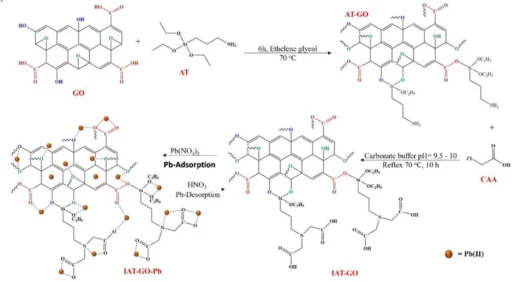
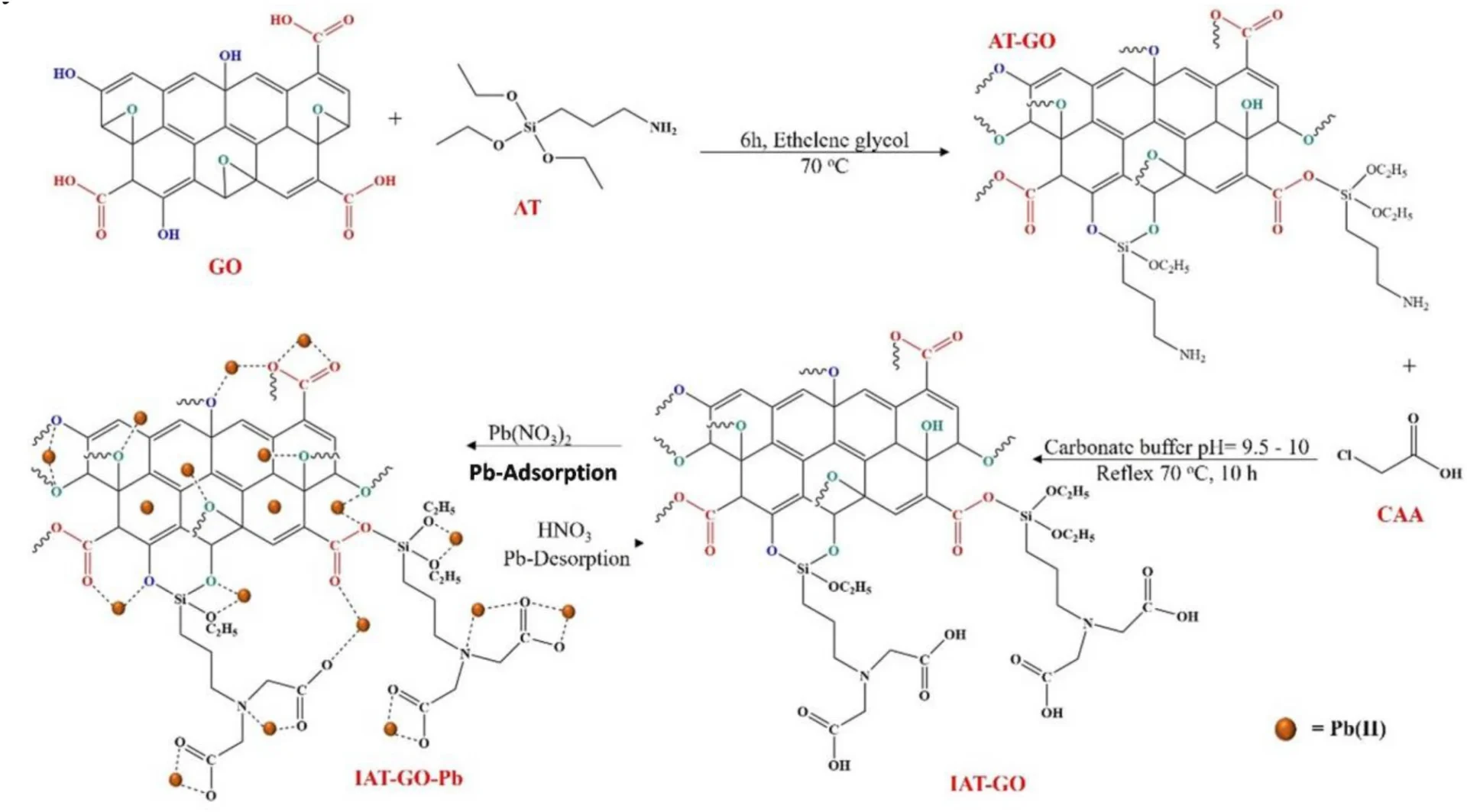
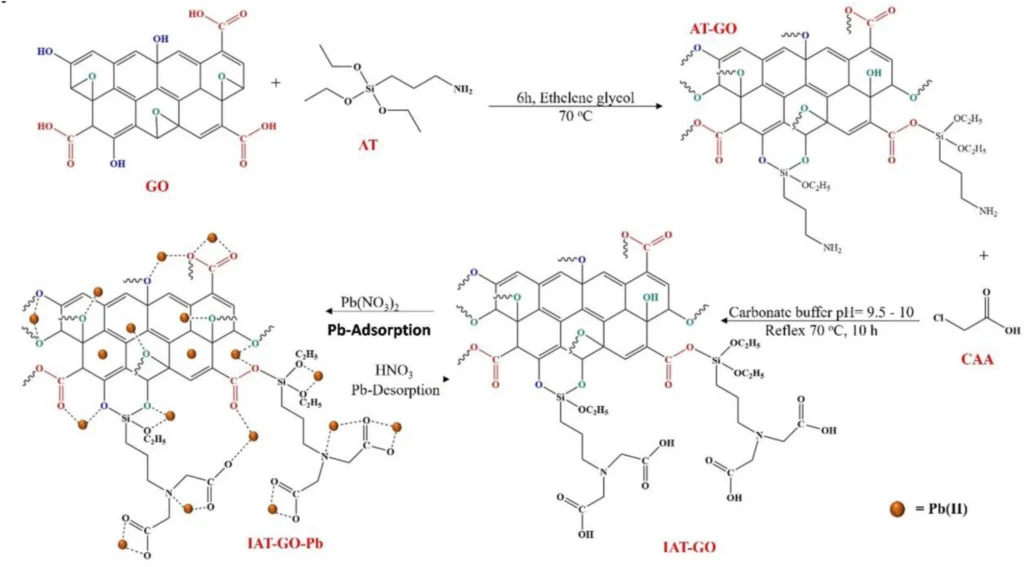
We added (3-iminodiacetic acid) propyltriethoxysilane (IDAPTES) to GO to make it better at getting rid of lead (II). IDAPTES is a chelating agent that can create powerful connections with metal ions, which makes it highly efficient for the process of adsorbing lead (II). During the functionalization process, IDAPTES molecules are attached to the surface of GO. This makes it better at absorbing and trapping lead (II) ions that are in polluted water.
We selected IDAPTES for functionalization because of its demonstrated effectiveness in chelating metal ions and compatibility with GO. The grafting of IDAPTES onto GO produces a substance called IDAPTES-GO, which exhibits a strong attraction to lead (II) ions. This enables the effective removal of lead(II) ions, even when they are present in low concentrations.
The preparation of propyltriethoxysilane graphene oxide (3-iminodiacetic acid) is currently underway:
Propyltriethoxysilane graphene oxide IDAPTES-GO is synthesized using a series of sequential procedures. Initially, we produce GO by subjecting graphite to a chemical oxidation procedure. After preparing the GO, we treat it with IDAPTES through a silanization reaction to make it functional. Graphene oxide (GO) is usually mixed with a suitable solvent, like ethanol, and then isocyanatopropyltriethoxysilane (IDAPTES) is added under carefully controlled conditions to make sure the grafting is consistent.
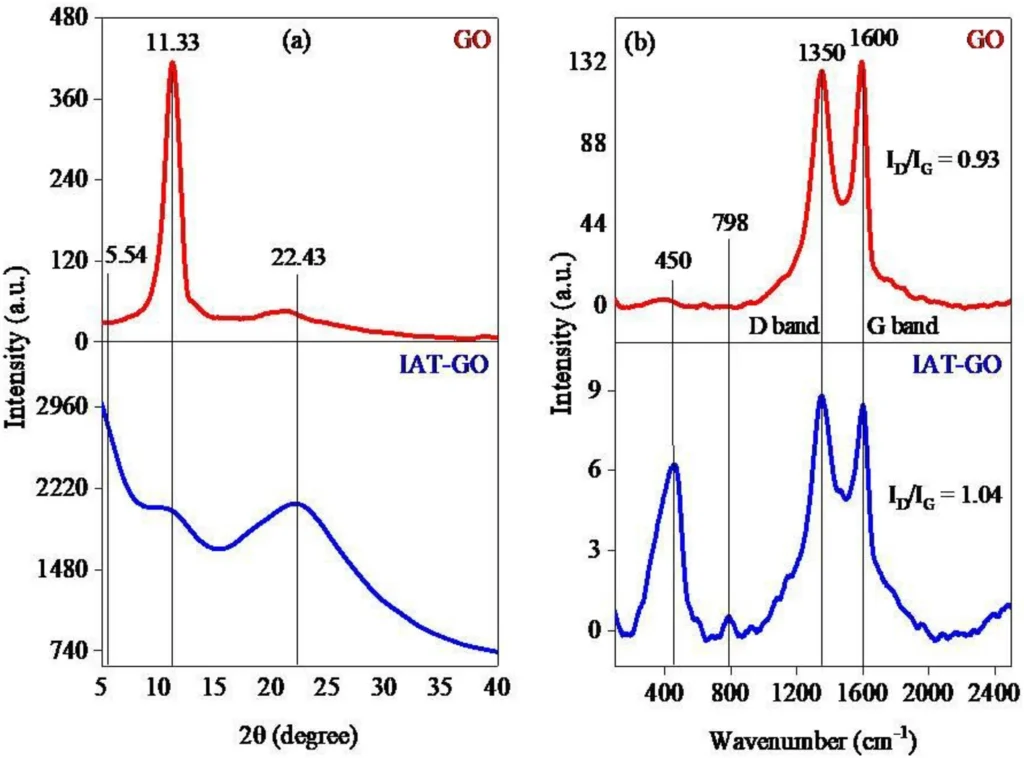
Characterizing the resulting Propyltriethoxysilane graphene oxide IDAPTES-GO is critical to verifying IDAPTES’ effective bonding and evaluating the material’s stability and adsorption properties. Fourier-transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM) are common ways to look at functionalized graphene oxide (GO) and make sure that IDAPTES is on its surface.
Lead(II) adsorption on functionalized graphene oxide (GO): an analysis of the underlying mechanism
IDAPTES-GO removes lead II using two primary methods: chelation and adsorption. For example, Propyltriethoxysilane graphene oxide IDAPTES has carboxyl and amine functional groups that can bind with lead(II) ions and make very stable complexes. The functional groups present on Propyltriethoxysilane graphene oxide IDAPTES-GO’s surface form binding sites that efficiently collect and immobilize lead (II) ions from water.
Several parameters, including pH, contact duration, and the initial concentration of lead (II) in the water, influence the adsorption process. The chelation mechanism is critical because it allows for the specific attachment of lead (II) while excluding other metal ions, resulting in Propyltriethoxysilane graphene oxide IDAPTES-GO’s high effectiveness in complicated water matrices.
Empirical Investigations and Findings:
Empirical investigations have conclusively shown that IDAPTES-GO is highly efficient in eliminating lead (II) from water. These investigations assessed the adsorption capacity, kinetics, and selectivity of IDAPTES-GO under various circumstances. We analyzed the data using adsorption isotherms, specifically the Langmuir and Freundlich models, to determine the maximum adsorption capacity of the material.
The findings showed that IDAPTES-GO has a strong ability to adsorb lead (II), often being better at it than other adsorbents. We determined the adsorption kinetics to be rapid, eliminating the bulk of lead(II) ions within the initial few minutes of contact. Additionally, IDAPTES-GO showed great selectivity for lead(II) even when other ions were present and could have caused problems. This suggests that it could be useful in real life.
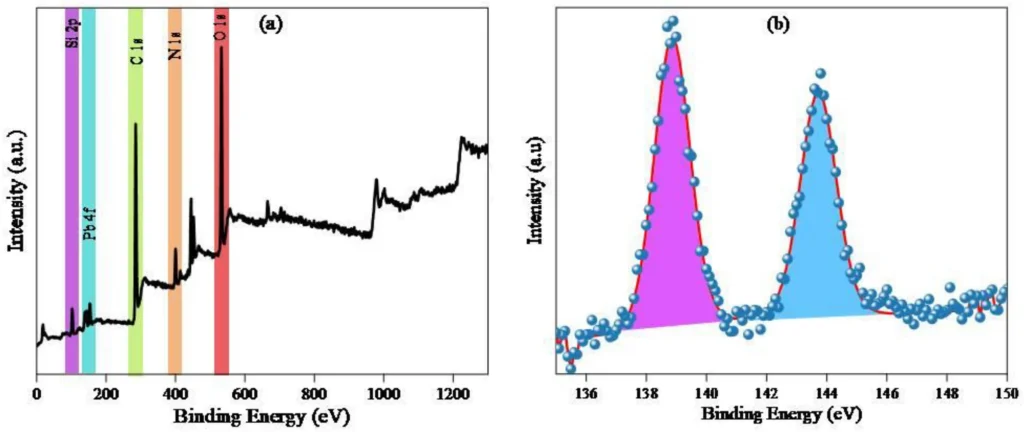
Improvements to the remediation process:
To effectively apply IDAPTES-GO in water cleanup, various aspects must be optimized. The factors that need to be considered include the water’s pH, contact time, temperature, and the starting concentration of lead (II). Research has shown that the pH level greatly influences IDAPTES-GO’s ability to adsorb substances, with mildly acidic environments typically exhibiting the most effective removal.
Higher temperatures often enhance the adsorption capacity by increasing the diffusion rates in the adsorption process. Nevertheless, the performance of the material under normal environmental conditions is crucial for practical applications. Furthermore, it is critical to limit the contact time required for effective lead removal (II) to ensure the practicality of implementing the procedure on a large scale.
The benefits of using IDAPTES-GO for lead (II) removal are as follows:
IDAPTES-GO has numerous benefits compared to conventional approaches for lead (II) elimination. The material’s high adsorption capability allows for the use of lesser quantities to accomplish the same level of remediation, resulting in cost and material usage reduction. Furthermore, IDAPTES-GO can regenerate and be reused multiple times without significantly reducing its efficiency, rendering it a sustainable option for water treatment.
The environmental impact of IDAPTES-GO is also positive. Because it is a nanomaterial, it has minimal toxicity and does not produce any harmful byproducts while undergoing the adsorption process. As a result, it becomes a more secure option compared to chemical treatments, which have the potential to introduce extra pollutants into the water.
Obstacles and Constraints:
While IDAPTES-GO has numerous benefits, some obstacles come with its widespread implementation. The production cost of graphene oxide and its functionalization with IDAPTES can be significant, which may limit its widespread use. Furthermore, it is necessary to conduct more research on the environmental consequences of nanomaterials, specifically regarding how they interact and behave in natural ecosystems, to guarantee their long-term safety.
Furthermore, implementing IDAPTES-GO in real-world situations poses technical challenges. It is necessary to address the uniform dispersion of the substance in water, prevent aggregation, and establish efficient techniques for recovering and recycling the material.
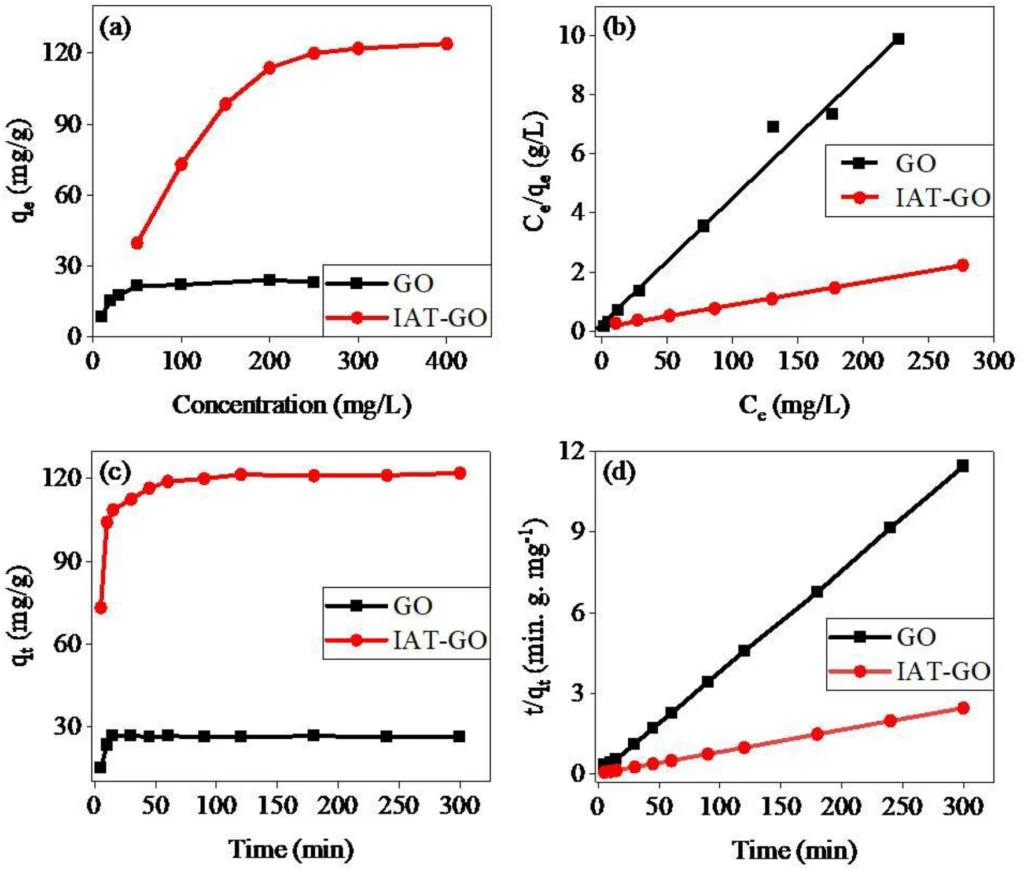
The study examines real-life examples and implements them in real-world scenarios:
Multiple case studies have shown the effective use of IDAPTES-GO for removing lead (II) in different environments. Regulatory authorities have effectively employed IDAPTES-GO in the treatment of industrial wastewater to reduce lead (II) levels below the prescribed limits. For enterprises facing strict environmental requirements, this provides a financially viable option.
Furthermore, IDAPTES-GO has the potential to be used in domestic water filtration systems, as well as in industrial applications. Because of its high efficiency and capacity to effectively eliminate low quantities of lead (II), it is considered an excellent choice for point-of-use filters, particularly in regions where lead contamination is a significant issue.
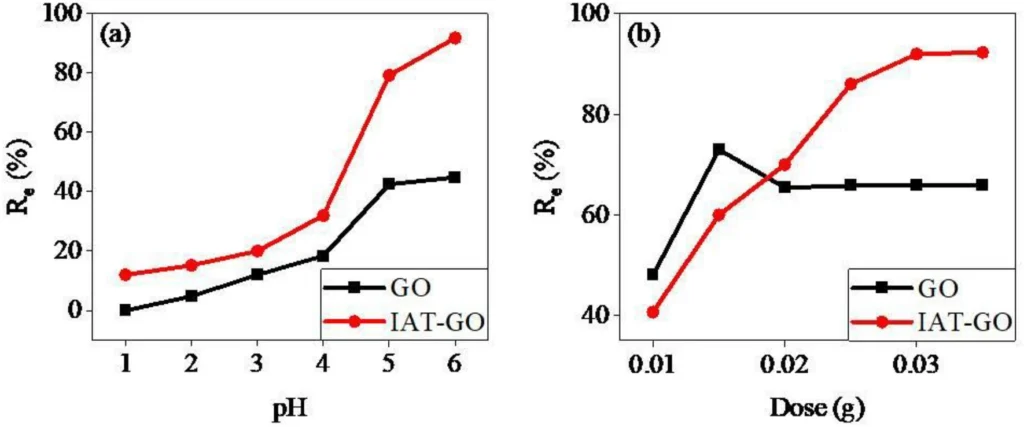
Prospects for the Future:
The prospects for water cleanup using graphene-based materials appear to be highly favorable. Researchers are currently focusing on enhancing the properties of graphene oxide (GO) and developing innovative functionalization techniques to effectively tackle a broader range of pollutants. We are currently researching to explore the potential of IDAPTES-GO in extracting additional heavy metals like mercury and cadmium.
Graphene-based materials are likely to find their way into various water treatment technologies, encompassing both large-scale industrial systems and small, portable devices, as the sector progresses. Sustained innovation and research will be crucial in fully harnessing the capabilities of these materials to guarantee the provision of safe and uncontaminated water for everyone.
In conclusion:
Overall, the process of modifying graphene oxide with (3-iminodiacetic acid) propyltriethoxysilane provides a remarkably efficient approach to treating lead-contaminated water (II). The ability of this sophisticated material to adsorb, selectively remove, and reuse makes it a promising solution to tackle the ongoing issue of lead (II) pollution in water. Despite the remaining obstacles in terms of cost and large-scale implementation, the potential of IDAPTES-GO to enhance the cleanliness and safety of water sources is unquestionable. As scientific research advances, IDAPTES-GO and similar substances have the potential to be extremely important in the future of water treatment.
Commonly Asked Questions (FAQs):
1). What factors contribute to the IDAPTES-GO’s effectiveness in removing lead (II)?
The functionalization of IDAPTES-GO with IDAPTES, which generates potent chelating sites for lead(II) ions, accounts for its effectiveness in removing lead(II). This ensures efficient adsorption, even at low concentrations.
2). What is the impact of lead (II) on human health?
Exposure to Lead(II) can result in significant health concerns, such as cognitive impairment, developmental delays, renal damage, and cardiovascular disorders, particularly in children and vulnerable groups.
3). Is IDAPTES-GO effective against other contaminants?
Indeed, IDAPTES-GO exhibits the capacity to effectively eliminate many heavy metals and pollutants, including mercury and cadmium. As a result, it can be classified as a versatile substance for water treatment.
4). Is it safe to use nanomaterials in water treatment?
While nanomaterials like IDAPTES-GO offer significant advantages, further research is necessary to evaluate their environmental impact and long-term safety, intending to reduce potential risks to ecosystems.
5). What are the potential future applications of graphene oxide in water remediation?
The prospects for utilizing graphene oxide in water remediation are promising, as current research is dedicated to improving its characteristics, broadening its uses, and incorporating it into diverse water treatment methodologies.
For more chemistry blogs, visit chemistry Master