Table of Contents
Opening statement:
Protein tyrosine phosphatases (PTPs) are essential for cellular signaling as they control the phosphorylation levels of different proteins, hence influencing a wide range of cellular functions. PTPN2 and PTPN1 are particularly intriguing because they play a significant role in crucial signaling pathways associated with immunological responses, metabolism, and cancer. Recently, the discovery of heterobifunctional degraders has opened up new ways to precisely control protein regulation, giving us a new way to work with protein complexes like PTPN2/N1. This article delves into the mechanistic understanding of how these degraders contribute to the formation of the PTPN2/N1 complex, providing insights into the structural and functional consequences of this interaction.
Comprehending Protein Tyrosine Phosphatases (PTPs):
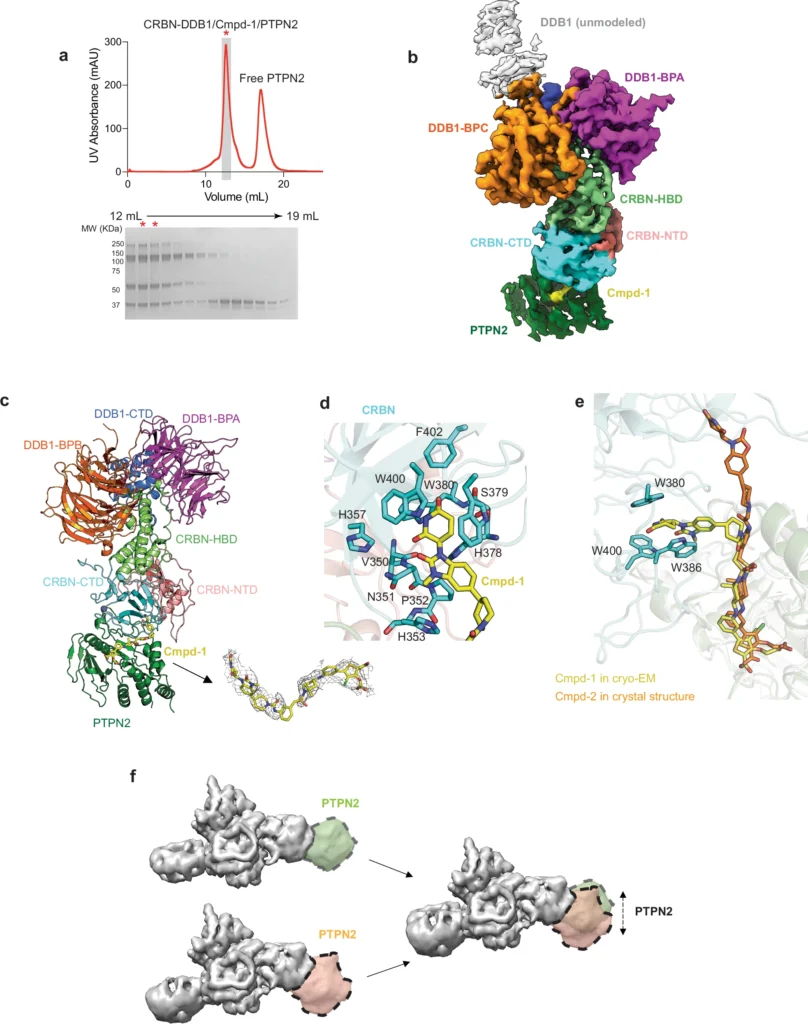
Protein tyrosine phosphatases are a class of enzymes that catalyze the removal of phosphate groups from phosphorylated tyrosine residues on proteins. They play a crucial role in regulating signal transduction pathways. PTPN2, also recognized as T-cell protein tyrosine phosphatase, and PTPN1, also known as PTP1B, are notable members of this family. PTPN2 regulates the immune system and has been associated with autoimmune diseases and cancer. PTPN1, on the other hand, is a crucial component in insulin signaling and metabolism regulation. Different clinical diseases link to their dysregulation, making them appealing candidates for therapeutic intervention. Heterobifunctional degrader design and degrader-induced PTPN2 complex formation.
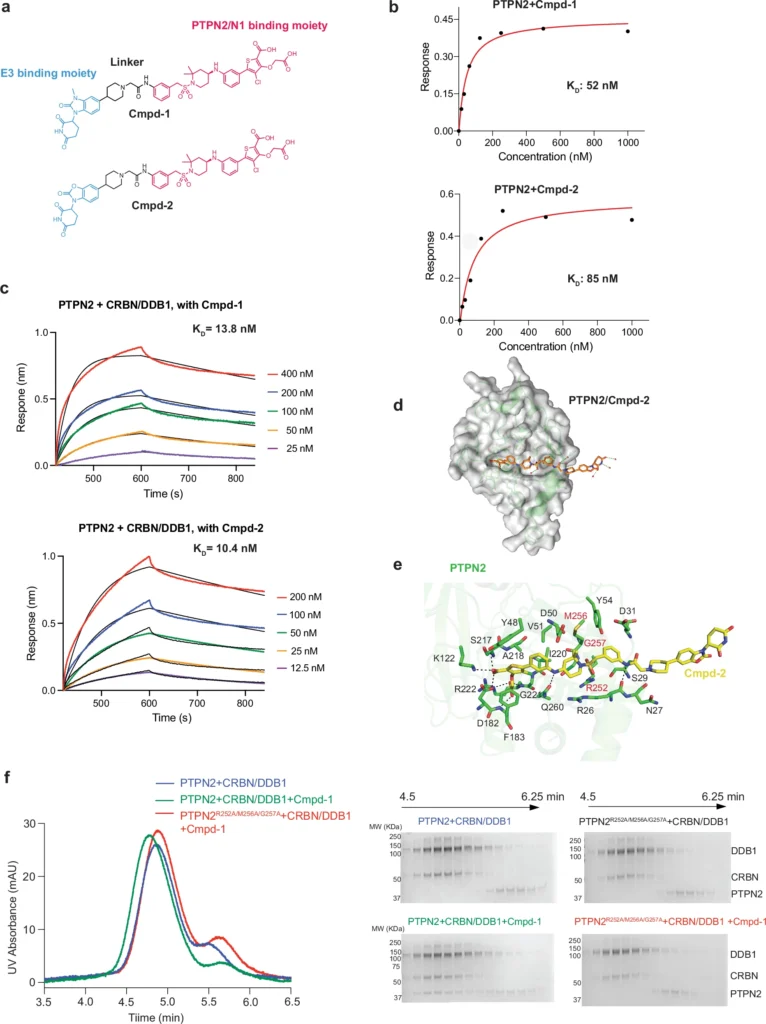
Heterobifunctional degraders represent a novel and promising area of drug discovery research:
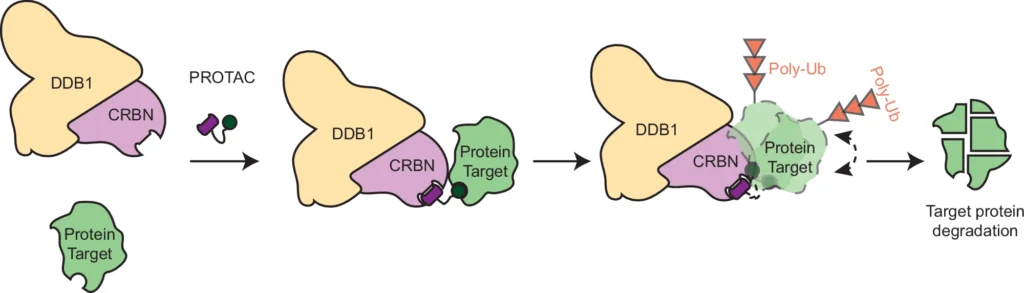
Small molecules known as heterobiofunctional degraders specifically target and break down target proteins. They achieve this by connecting the target protein to an E3 ubiquitin ligase, which triggers a process called ubiquitination. This process ultimately leads to the degradation of the target protein by the proteasome. Contrary to conventional inhibitors that simply obstruct the function of specific proteins, these degraders completely eradicate the target protein from the cell. This method has demonstrated significant potential in selectively targeting proteins that were previously considered “undruggable,” hence broadening the range of therapeutic opportunities.
An introduction to the PTPN2/N1 complex:
The connection between PTPN2 and PTPN1 is an interesting occurrence in cellular signaling. Although both proteins have structural similarities, they perform diverse functions in separate signaling pathways. The PTPN2/N1 complex must be made in order to control the signaling pathways that manage immune responses, glucose metabolism, and cell growth. Understanding the interactions and impact of these proteins on each other’s function provides valuable insight into their wider physiological and pathological roles.
Understanding the Mechanism of Complex Formation Induced by Heterobifunctional Degraders:
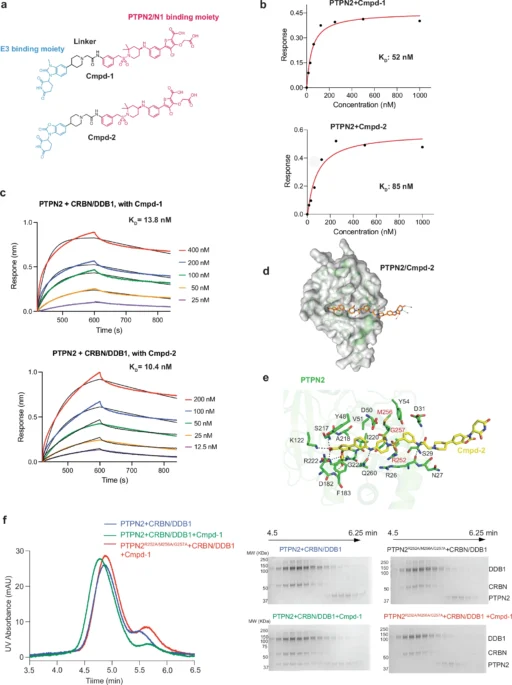
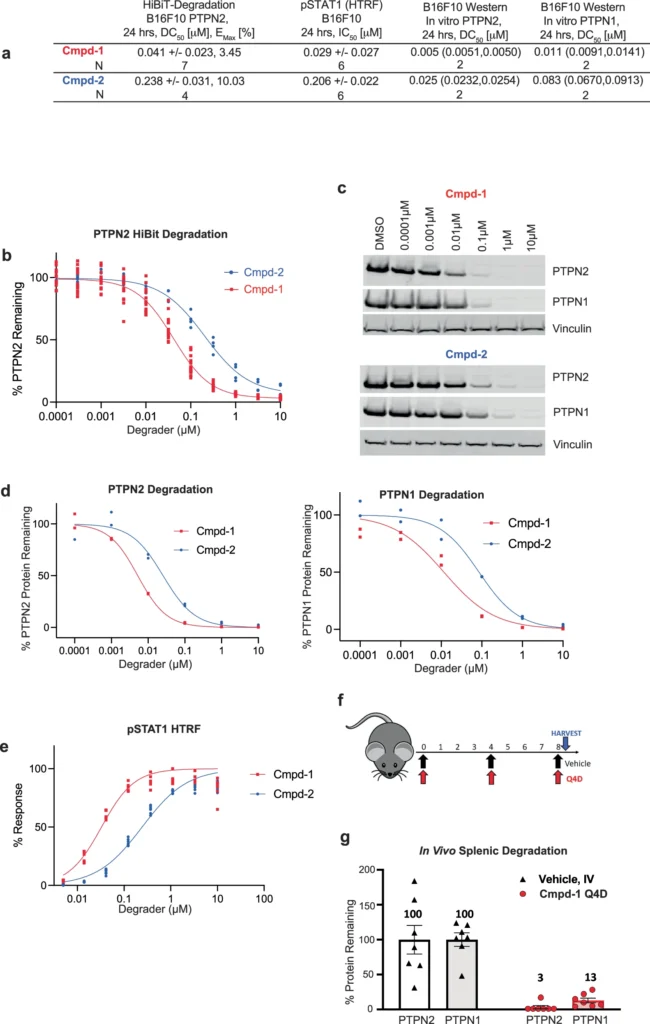
Heterobifunctional degraders enhance the interaction between PTPN2 and PTPN1 by simultaneously binding to both proteins, thus favoring the formation of a complex that may not ordinarily occur. The degrader facilitates the creation of this intricate structure by connecting specific regions on PTPN2 and PTPN1, aligning them in a manner that promotes interaction. Structural analyses show that this enforced closeness alters the structural arrangements of both proteins, potentially resulting in functional modifications that affect subsequent signaling pathways. Biological activity of Cmpd-1 and Cmpd-2.
Analysis of the PTPN2/N1 complex provides valuable information about its structural characteristics:
The PTPN2/N1 complex has been seen at atomic resolution using advanced techniques in structural biology, including X-ray crystallography and cryo-electron microscopy (cryo-EM). These investigations emphasize the crucial residues and domains that facilitate the interaction, specifically the catalytic domains that are accountable for phosphatase activity. We detect slight changes in conformation, especially in the active sites, when the heterobifunctional degrader binds. This indicates that the complex creation may affect the enzymatic activity of PTPN2 and PTPN1.
The formation of the PTPN2/N1 complex holds significant functional implications:
The PTPN2/N1 complex assembly has significant implications for cellular signaling. By introducing these intricate, heterobifunctional degraders, it is possible to manipulate the enzymatic functions of both PTPs, resulting in changes in the subsequent signaling cascades. For example, breaking down this intricate structure could improve insulin signal transmission by decreasing the inhibitory effect of PTPN1, thereby providing therapeutic advantages for illnesses such as type 2 diabetes. Moreover, the manipulation of the complex could have an effect on immunological signaling, thus offering therapeutic possibilities for autoimmune illnesses.
The ubiquitin-proteasome system plays a crucial role in the degradation of complex molecules:
The ubiquitin-proteasome system plays a crucial role in the functioning of heterobifunctional degradations. After the PTPN2/N1 complex forms, it serves as a substrate for ubiquitination, which designates it for destruction by the proteasome. This deterioration not only decreases the levels of PTPN2 and PTPN1, but also interrupts their signaling functions, resulting in notable physiologic consequences. The effectiveness of this process is vital for the success of degrader-based therapeutics since it determines the degree to which target proteins are removed from the cell. Cryo-EM structure of the degrader-induced PTPN2/CRBN-DDB1 complex.
Targeting the PTPN2/N1 Complex: Therapeutic Benefits
Utilizing heterobifunctional degraders to target the PTPN2/N1 complex has significant therapeutic promise. Approaches that specifically break down these proteins may improve conditions characterized by abnormal PTPN2 or PTPN1 activity, such as cancer, diabetes, and autoimmune illnesses. By adjusting the activity of these PTPs, degraders provide a more precise method of treating diseases, reducing the negative effects commonly associated with broad-spectrum inhibitors.
Difficulties in Targeting the PTPN2/N1 Complex:
Although heterobifunctional degraders show potential, there are still significant obstacles to overcome when it comes to targeting the PTPN2/N1 complex. The selectivity of these degraders is an important issue to consider, as non-target effects could result in undesirable outcomes. Furthermore, it is essential to optimize the stability and bioavailability of degraders in order to achieve optimal drug delivery and maintain a sustained therapeutic impact. Another challenge in the development of these medicines is to address the emergence of resistance mechanisms, which can occur through mutations or compensatory pathways.
Analysis of Specific Instances: Insights from Both Preclinical and Clinical Research
Multiple preclinical models have shown that heterobifunctional degraders effectively target the PTPN2/N1 complex. These investigations have demonstrated substantial decreases in disease indicators and enhanced results in models of cancer and metabolic illnesses. Clinical trials are currently underway to evaluate the safety and efficacy of these degraders in patients. Preliminary findings show considerable promise, emphasizing the potential of this method to completely transform the treatment of illnesses caused by abnormal PTP activity.
Advanced methodologies for investigating protein-protein interactions:
Using advanced techniques like co-immunoprecipitation, fluorescence resonance energy transfer (FRET), and surface plasmon resonance (SPR) has made it easier to study the connection between PTPN2 and PTPN1. These techniques enable the accurate analysis of protein-protein interactions, yielding crucial information on the strength of binding and the speed at which complex formation occurs. Advancements in these methods consistently improve our understanding of interactions caused by degraders, thereby assisting in the development of more potent medicinal compounds.
A Comparative Analysis: Heterobifunctional Degraders vs. Traditional Inhibitors
Heterobifunctional degraders have multiple advantages over typical inhibitors when it comes to targeting the PTPN2/N1 complex. Degraders not only inhibit the action of these proteins but also eradicate them from the cell, resulting in a longer-lasting and more powerful impact. However, the choice between degraders and inhibitors is contingent upon the specific circumstances of the illness and the intended treatment outcome. Case studies demonstrate instances where degraders surpass inhibitors, especially when complete elimination of proteins is required for therapeutic effectiveness. Schematic representation of bifunctional degrader-mediated ubiquitination of a protein of interest by CRBN-DDB1.
The future prospects and areas for further research are worth exploring:
Research is currently underway to enhance the design of heterobifunctional degraders and expand their therapeutic applications in targeting the PTPN2/N1 complex, suggesting a promising future for these compounds. The combination of degraders and conventional treatments has the potential to improve effectiveness and overcome resistance. We anticipate that upcoming technologies, such as proteolysis-targeting chimeras (PROTACs) and molecular glues, which degrade proteins, will enhance this area of study by offering novel approaches to target difficult proteins.
In conclusion:
Our understanding of how heterobifunctional degraders create the PTPN2/N1 complex improves our understanding of how we might use these novel compounds to control important signaling pathways. Degraders facilitate the formation of a complex between PTPN2 and PTPN1, resulting in significant functional alterations that have potential therapeutic value. Advancements in research are leading to the creation of more sophisticated degraders, which have the potential to significantly transform the treatment of disorders related to PTP dysregulation. This development brings optimism for the possibility of improved and precisely focused therapeutics.
Frequently Asked Questions:
1). What are heterobifunctional degraders?
Small molecules called heteroobifunctional degraders target and break down target proteins by linking them to an E3 ubiquitin ligase. This causes the proteins to be ubiquitinated and then broken down by proteasomes.
2). What is the effect of the PTPN2/N1 complex on cellular signaling?
The PTPN2/N1 complex controls signaling pathways that are involved in metabolism, immune responses, and cell growth. This is very important for diseases like diabetes and cancer.
3). What are the benefits of utilizing heterobifunctional degraders instead of conventional inhibitors?
Degraders, in contrast to typical inhibitors, have the ability to not only inhibit target proteins but also trigger their breakdown, resulting in a more comprehensive and long-lasting therapeutic impact.
4). What are the primary obstacles in the development of heterobifunctional degraders?
The challenges involve the need to ensure specificity in order to prevent off-target effects, optimize stability and bioavailability, and overcome potential resistance mechanisms.
5). What impact could these discoveries have on future medication discoveries?
These discoveries provide a foundation for the creation of more potent targeted treatments, particularly for disorders that include proteins that are challenging to target. We can achieve this by leveraging the unique mechanisms of heterobifunctional degraders.
For more chemistry blogs, visit chemistry Master