Table of Contents
Regioselective hydroformylation is an important idea in organic chemistry that lets you precisely change the regiochemistry of making aldehydes from olefins. Among the catalyst systems designed for this purpose, rhodium-zeolite catalysts demonstrate favorable characteristics, including excellent regioselectivity and activity. Rhodium zeolite facilitates regioselective hydroformylation, which is the subject of this comprehensive investigation. It seeks to clarify the mechanism, applications, benefits, difficulties, and environmental consequences of this process.
Overview of Regioselective Hydroformylation:
a). Regioselective hydroformylation: Regioselective hydroformylation is a process that involves selectively adding a formyl group to a specific region of a molecule.
Regioselective hydroformylation is the process of adding a formyl group (-CHO) to a certain spot on an unsaturated hydrocarbon, usually an olefin, to make aldehydes that are either linear or branching. This procedure is crucial in the production of different organic compounds, where the ability to manage the regiochemistry of the reaction guarantees the intended outcome of the product.
b). Regioselectivity’s importance in organic synthesis:
Regioselectivity is a critical factor in organic synthesis, allowing chemists to precisely modify the structure and functioning of molecules. Regioselective hydroformylation entails selectively directing the formyl group’s attachment to a specific carbon atom of the olefin substrate. This process enables the production of complex compounds and medicinal intermediates.
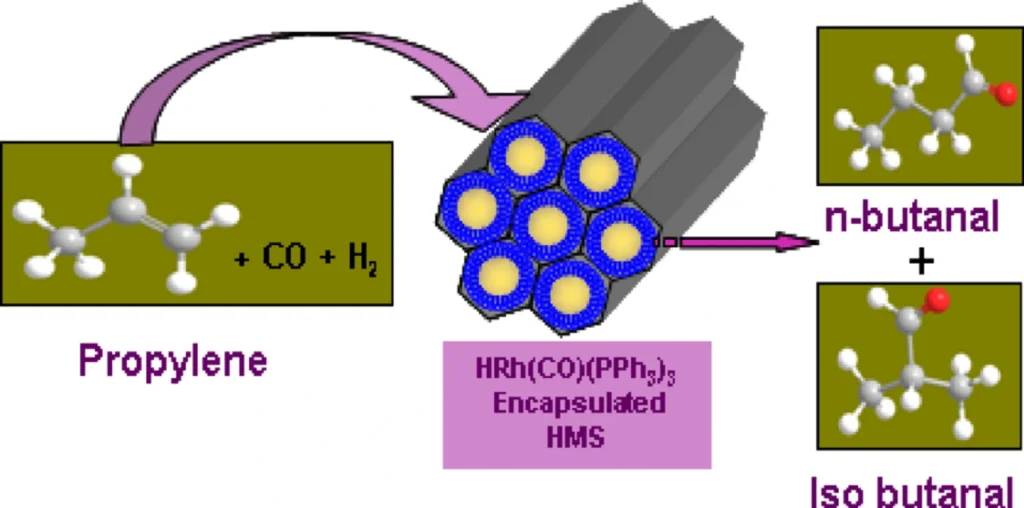
Regioselective hydroformylation catalysts:
a). The importance of catalysts in hydroformylation reactions:
Catalysts are very important for improving hydroformylation processes because they lower the activation energy barrier and help make the aldehydes that are wanted. They improve the selectivity, efficiency, and pace of the reaction, rendering it economically feasible for industrial applications.
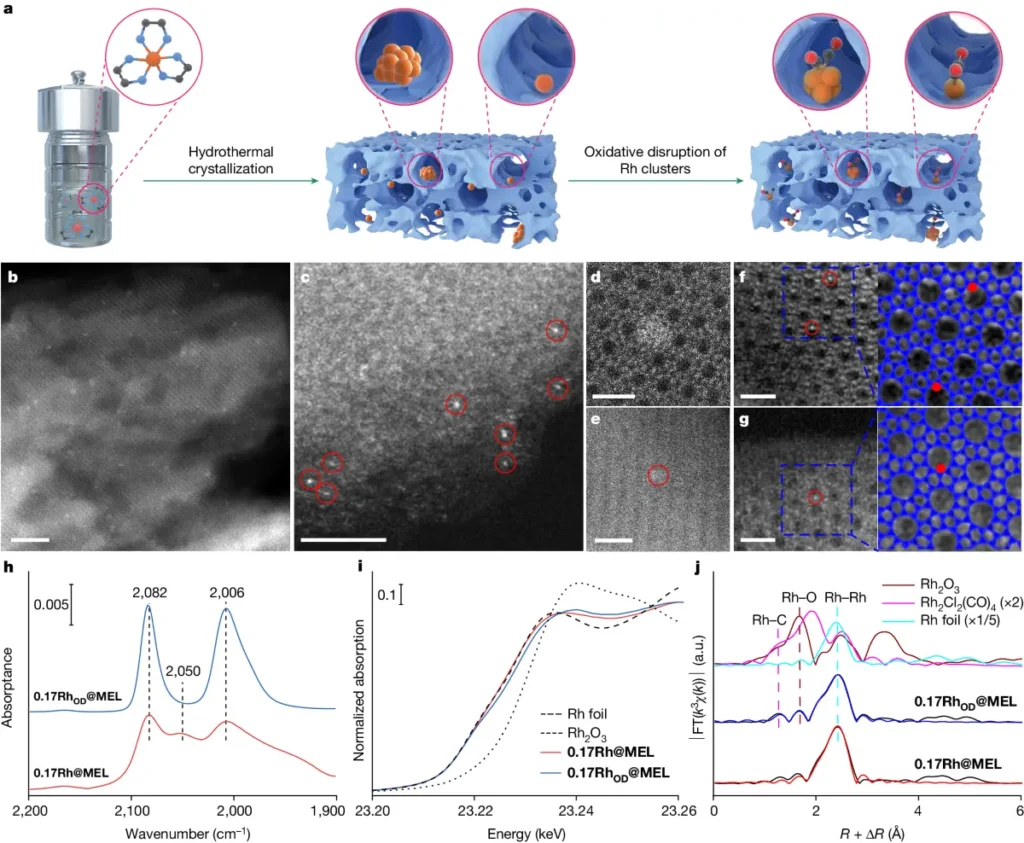
b). Properties of Rhodium-Zeolite Catalysts:
Rhodium-zeolite catalysts are a type of heterogeneous catalyst made up of rhodium nanoparticles that are held in place by zeolite matrices. These catalysts are great for regioselective hydroformylation processes because they have unique properties like a large surface area, pores that can be adjusted in size, and high heat stability.
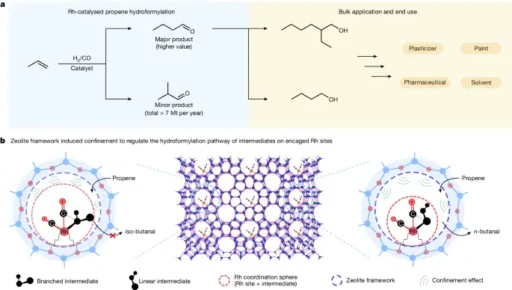
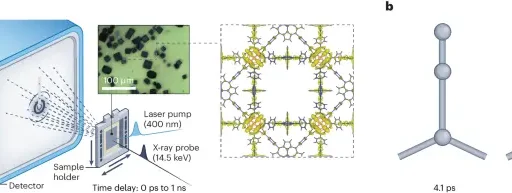
Regioselective hydroformylation with rhodium-zeolite: Detailed procedure Elucidation of the Reaction Mechanism:
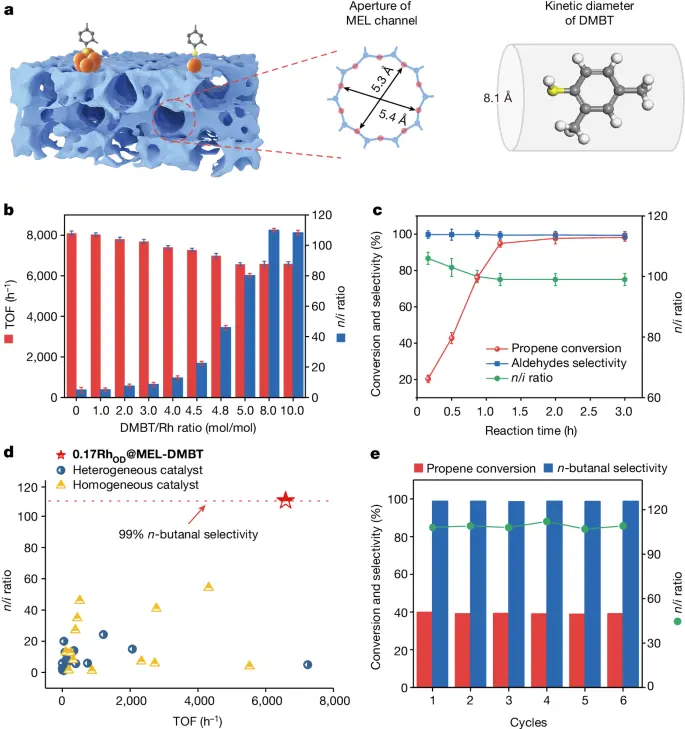
A rhodium-zeolite catalyst facilitates the selective hydroformylation of propene towards a specific region. This process occurs in a sequence of clearly defined stages.
1). Adsorption of Reactants on the Catalyst Surface: Propene molecules and syngas, a mixture of carbon monoxide and hydrogen, attach to the active sites of the rhodium nanoparticles supported on the zeolite surface during the adsorption process.
2). Selective Activation of Propene Molecules: Rhodium species on the catalyst surface selectively activate propene molecules, facilitating their attachment to the rhodium center to form a rhodium-alkyl intermediate.
3). Hydroformylation Reaction: The hydroformylation reaction occurs when the rhodium-alkyl intermediate reacts with syngas, resulting in hydroformylation. This process produces both linear and branched aldehydes, with a strong preference for specific regioselectivity.
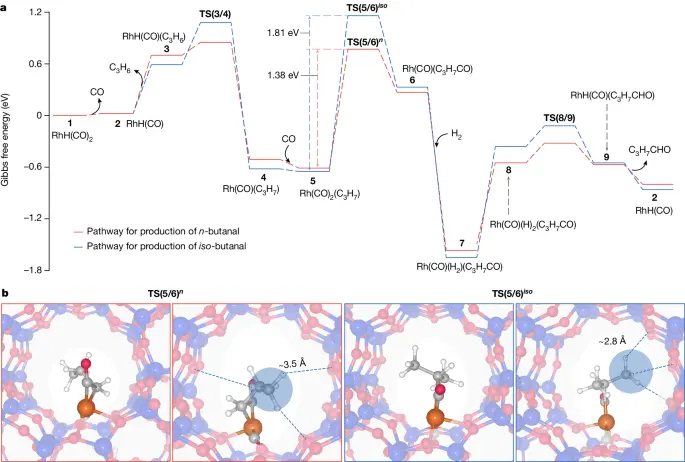
Variables Affecting Regioselectivity:
Many parameters influence the regioselectivity of hydroformylation processes catalyzed by rhodium-zeolite catalysts.
1). Catalyst Structure and Composition: The size, shape, and distribution of rhodium nanoparticles on the zeolite support in the catalyst structure and composition significantly influence the regioselectivity of the reaction.
2). Reaction Conditions: The reaction conditions, including temperature, pressure, and reactant concentrations, are important factors that influence the hydroformylation reaction’s regiochemistry. These parameters impact the adsorption and activation energies of the reactants.
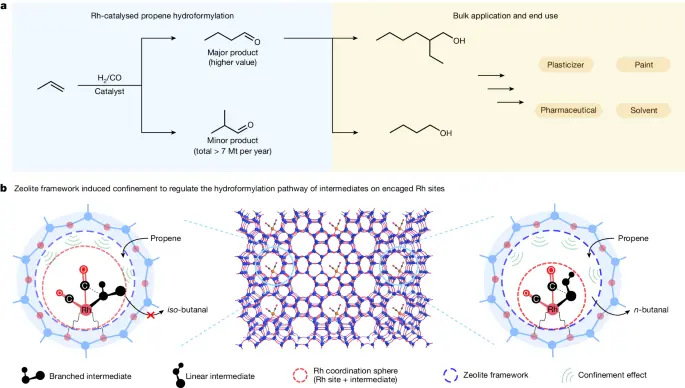
Applications of Regioselective Hydroformylation:
There are both industrial and academic applications of regioselective hydroformylation.
Various industrial and academic contexts widely employ rhodium-zeolite catalysts for regioselective hydroformylation.
1). Industrial and Academic Applications:
a). Fine Chemical Synthesis: The capacity to selectively add formyl groups to olefins allows for the efficient production of fine chemicals, medicines, and agrochemicals with customized characteristics and functionalities.
b). Scholarly Research: Regioselective hydroformylation is a useful method in academic research for looking into how reactions work, making new catalytic systems, and building complex structures with accurate regiochemistry.
2). Synthetic Routes to Valuable Intermediates: Methods for producing valuable intermediate compounds through synthetic routes
Regioselective hydroformylation makes it possible to create a wide range of aldehyde derivatives with unique regiochemistry. These are important steps in the creation of many organic molecules, including medicines, flavors, and fragrances.
Advantages of Rhodium-Zeolite Catalysts:
1). High Regioselectivity and Activity: Rhodium-zeolite catalysts benefit from their exceptional regioselectivity and activity.
Rhodium-zeolite catalysts provide remarkable selectivity and efficiency in hydroformylation processes, allowing for fine manipulation of the aldehyde production position and high yields of desired products.
2). Potential for Reusability and Recycling:
Rhodium-zeolite catalysts have a variety of parts that make it easy to remove them from reaction mixtures and put them back together again. This makes them highly suitable for repeated usage and recycling in numerous reaction cycles. This attribute improves the long-term viability and efficiency of regioselective hydroformylation procedures.
Obstacles and Prospects for the Future:
a). Enhancing Catalyst Performance:
Additional investigation is required to boost the efficiency of rhodium-zeolite catalysts for regioselective hydroformylation. This research should primarily concentrate on refining the structure and composition of the catalyst to improve regioselectivity, activity, and stability.
b). Advancement of Catalytic Systems for Long-term Sustainability:
The focus should be on creating catalytic systems and reaction conditions that are sustainable and minimize waste production, energy usage, and environmental harm. At the same time, these systems should maximize selectivity and efficiency in regioselective hydroformylation processes.
Examples of real-life situations and accomplishments:
1). Prominent examples of effective responses:
Company X: Company X has achieved success in developing a regioselective hydroformylation process. This process utilizes rhodium-zeolite catalysts to manufacture a crucial intermediate for a new pharmaceutical molecule. This technology’s successful development demonstrates its potential for commercial applications.
Research Group Y: Using rhodium-zeolite catalysts, Research Group Y did complex spectroscopic and computational studies to figure out how regioselective hydroformylation works. Their research significantly contributed to the fundamental knowledge of the reaction and the concepts behind catalyst design.
2). Effects on the Chemical Industry:
The use of rhodium-zeolite catalysts in regioselective hydroformylation has had a significant impact on the chemical industry. This advancement allows for the efficient production of intricate compounds and pharmaceutical intermediates with customized regiochemistry and enhanced sustainability.
Implications for the environment and economy:
1). We must consider factors related to sustainability:
Rhodium-zeolite catalysts have the potential to provide environmental advantages in regioselective hydroformylation. The catalysts achieve this by minimizing waste production, lowering energy usage, and mitigating the formation of toxic by-products commonly associated with conventional homogeneous catalytic systems.
2). Cost-effectiveness analysis of regioselective processes:
Rhodium-zeolite catalysts are very good at regioselectivity and effectiveness. They lower costs by reducing unwanted reactions and increasing the production of desired products. This makes regioselective hydroformylation processes more economically viable.
In conclusion:
Rhodium-zeolite is used as a catalyst for the hydroformylation of propene. This selective formation of aldehyde intermediates makes the process very flexible and useful for synthesis. Due to their excellent regioselectivity, activity, and recycling ability, rhodium-zeolite catalysts show considerable potential for enhancing sustainable and selective organic synthesis.
Frequently Asked Questions:
1). What is regioselective hydroformylation?
Regioselective hydroformylation is a chemical reaction that selectively adds a formyl group to a specific region of a molecule. When a formyl group (-CHO) is added to a certain spot on an unsaturated hydrocarbon, usually an olefin, through a chemical process called regioselective hydroformylation, linear or branched aldehydes are made.
2). What are rhodium-zeolite catalysts?
Rhodium-zeolite catalysts consist of rhodium nanoparticles supported on zeolite materials. Rhodium-zeolite catalysts consist of rhodium nanoparticles supported on zeolite matrices. They are classified as heterogeneous catalysts. They demonstrate a notable tendency to selectively react in specific regions and display significant effectiveness in hydroformylation processes.
3). Which variables affect the regioselectivity of hydroformylation reactions?
The regiochemistry of hydroformylation processes is significantly influenced by the structure and composition of the catalyst, as well as the reaction parameters, including temperature, pressure, and reactant concentrations.
4). What are the purposes of regioselective hydroformylation?
Regioselective hydroformylation synthesizes fine chemicals, medicines, and specialty chemicals. It allows for the efficient production of specific intermediates with customized regiochemistry, thereby facilitating the creation of important compounds.
5). We are investigating the environmental and economic consequences of regioselective hydroformylation using rhodium-zeolite catalysts.
Rhodium-zeolite catalysts have the potential to provide environmental benefits by reducing waste production and energy consumption. Additionally, their strong regioselectivity and activity contribute to cost-effectiveness and improved economic feasibility.
For more chemistry blogs, visit chemistry Master