Table of Contents
Overview of Resonant Intermolecular Coulombic Decay:
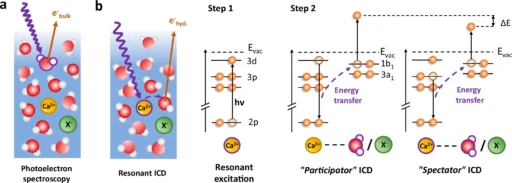
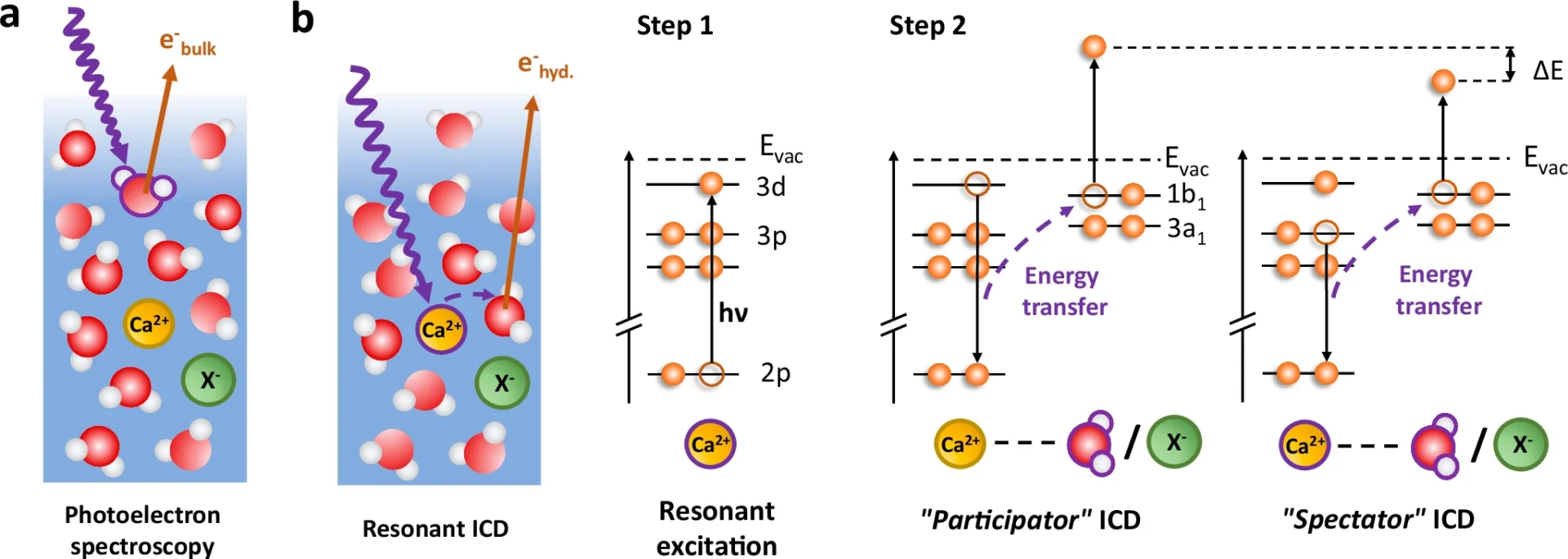
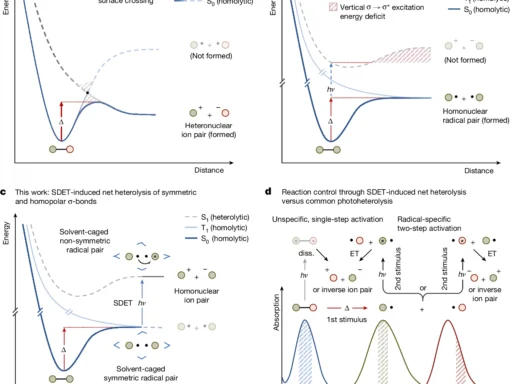
The solvation shell is one of chemistry’s most intriguing concepts. The solvent forms a protective layer around the molecules, acting as a crucial component in numerous chemical reactions. However, how can we effectively investigate a subject that is both difficult to grasp and constantly changing? Resonant intermolecular Coulombic decay (RICD) is a phenomenon that offers a distinct perspective on the complex mechanisms of the solvation shell. The point of this article is to give a full explanation of how RICD (Resonance-Enhanced Inelastic X-ray Scattering) helps us study important structures and find useful information that could lead to huge steps forward in fields like drug production and catalysis. Schematic diagram of resonant ICD processes in solvated calcium.
What is a Solvation Shell?:
Before fully comprehending the enchantment of Resonant Intermolecular Coulombic Decay, we must acquire a thorough understanding of the solvation shell in its entirety. Consider a molecule immersed in a solvent, such as water. The solvent molecules tend to aggregate around the solute, creating a protective coating. The solvation shell, also known as the hydration layer, is crucial in influencing the interaction between the solute and its surroundings.
The solvation shell plays an active role in influencing chemical characteristics and reactions, rather than simply being a passive observer. For instance, the solvation shell controls the behavior of enzymes, the process of salt dissolution, and the stability of complex compounds. Chemists have been eager to uncover this subtle yet potent force.
Solvation Dynamics:
How is a solvation shell formed? The central focus is on attractiveness. The solute and solvent molecules interact through a variety of forces, including electrostatic, van der Waals, and hydrogen bonding, resulting in this shell. Various factors, including the characteristics of the solute and solvent, temperature, pressure, and the presence of other solutes, influence the composition and behavior of a solvation shell.
Once established, the solvation shell is not stationary. The system is in a state of perpetual change, as solvent molecules are constantly entering and exiting the shell. This behavior’s dynamic nature has a significant impact on chemical reactions. In certain situations, the solvation shell can stabilize transition states in a reaction, thereby reducing the energy barrier and accelerating the process.
An Overview of the Intermolecular Coulombic Decay (ICD):
Having established the context, let us now introduce the concept of intermolecular Coulombic decay (ICD). We first observed ICD, or Interatomic Coulombic Decay, in the early 2000s. It involves the transfer of excess energy from one excited atom or molecule to a nearby molecule, resulting in ionization. This non-radiative process plays a crucial role in energy transfer mechanisms, particularly in loosely bound systems such as van der Waals complexes.
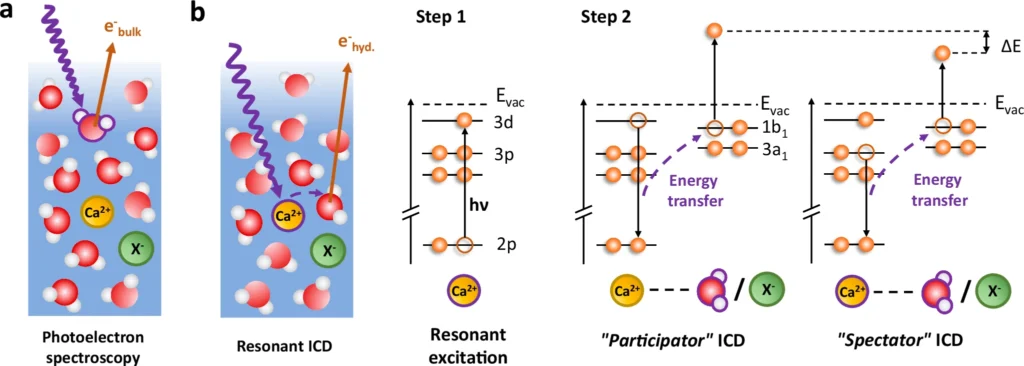
ICD, or Interatomic Coulombic Decay, has garnered significant attention in the realm of chemical physics due to its ability to offer a novel approach to investigating molecular interactions. Various fields utilize it, from studying the effects of radiation on living tissues to creating novel materials with targeted electrical characteristics. Resonant absorption and decay of solvated Ca2+.
Resonant Intermolecular Coulombic Decay (RICD):
Expanding on the concept of ICD, we have a phenomenon known as resonant intermolecular Coulombic decay (RICD). Resonance occurs when the energy of the initial excitation aligns with the energy difference between two states of the system. The resonance state greatly improves the efficiency of the decay process, making RICD a potent tool for investigating molecular interactions.
The resonance condition forms the primary distinction between ICD and Resonant Intermolecular Coulombic Decay. ICD can manifest in several systems, but Resonant Intermolecular Coulombic Decay is more discerning, necessitating a precise alignment between the excitation energy and the internal energy levels of the system. RICD’s selectivity makes it very valuable for investigating intricate and intricate surroundings, such as solvation shells.
Using Resonant Inelastic X-ray Scattering (RICD), investigate the Solvation Shell:
RICD provides novel opportunities for investigating solvation shells. Researchers can use resonant inelastic X-ray scattering (RICD) to get very accurate information about the make-up and movement of solvation shells by carefully adjusting the excitation energy. RICD, or Resonance-Enhanced Infrared Circular Dichroism, can provide insights into the spatial organization of solvent molecules around a solute, the dynamics of this arrangement, and the impact of various solvents on the solvation process.
Researchers routinely apply experimental techniques like photoelectron spectroscopy to observe RICD in action. These experiments include stimulating a solute within its solvation shell and monitoring the resultant electrons or ions. Through particle analysis, scientists can determine the solvation shell’s arrangement and interactions.
Theoretical Models of RICD in Solvation Shells:
Understanding Resonant Intermolecular Coulombic Decay necessitates not only doing research but also developing sturdy theoretical models. In this context, quantum mechanics is critical because it provides the necessary tools for simulating and predicting RICD processes. Scientists can use computational simulations, such as density functional theory (DFT), to investigate the behavior of Resonant Intermolecular Coulombic Decay in various solvation situations.
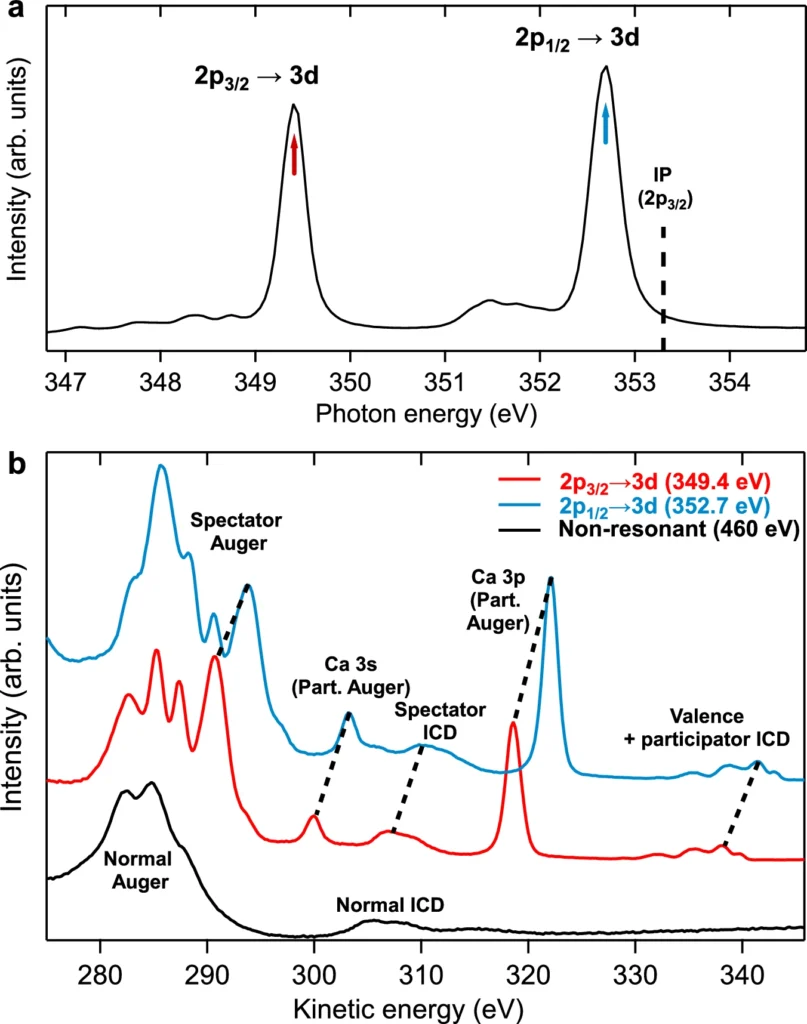
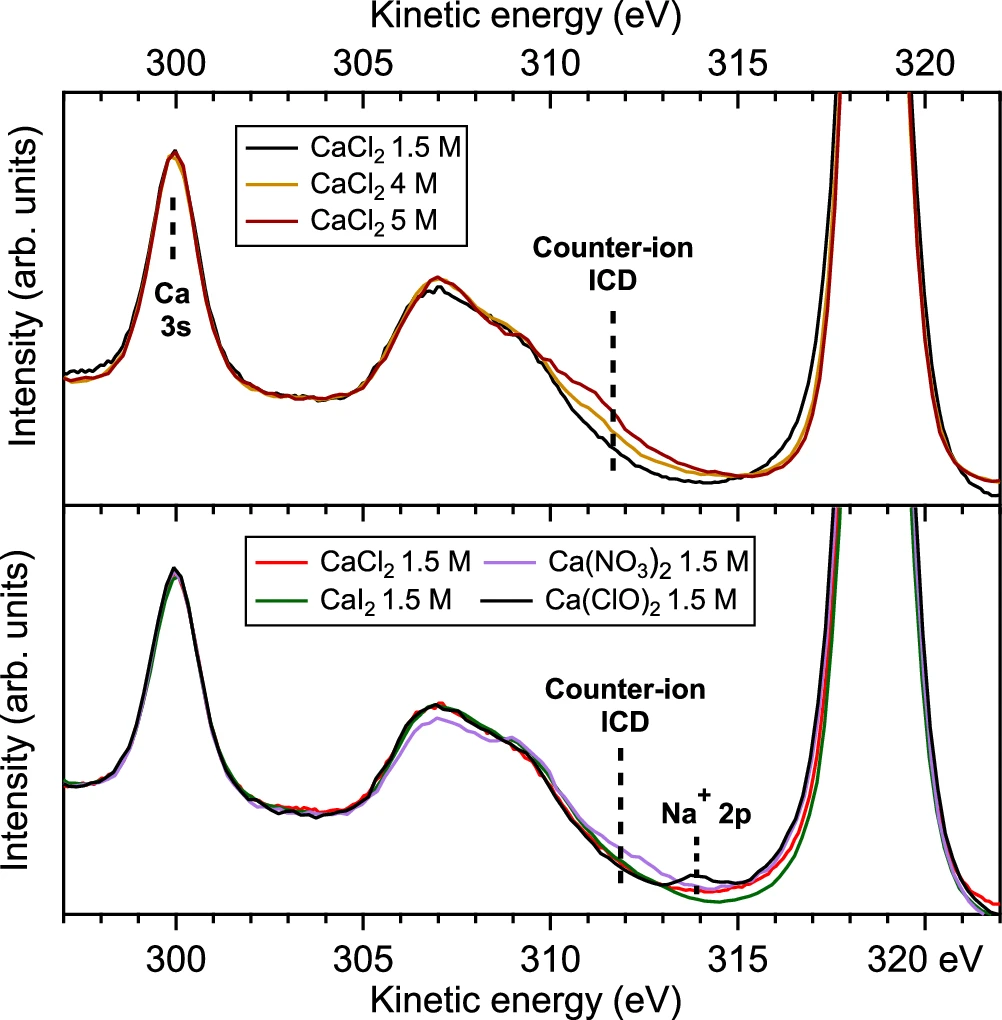
These models are essential for analyzing experimental results and formulating future studies. They help forecast the potential impact of changes in the solute, solvent, or experimental conditions on the Resonant Intermolecular Coulombic Decay process. The precision of these models steadily increases as processing capacity and our understanding of quantum interactions improve. Observation of a signature for ion pairing in the spectator ICD spectra at 349.4 eV (main 2p3/2 → 3d line, see Fig. 2) for several calcium salt solutions.
Observations of Resonance-Induced Charge Delocalization in Solvation Shells:
Several critical experiments have shed light on how Resonant Intermolecular Coulombic Decay functions within solvation shells. For instance, studies on water clusters have demonstrated the application of Resonant Intermolecular Coulombic Decay in examining the arrangement of water molecules around a core ion. These experiments have yielded fundamental insights into the composition of hydration shells, which are essential for comprehending phenomena such as ion transportation in biological systems.
Nevertheless, quantifying Resonant Intermolecular Coulombic Decay is a challenging endeavor. The process is extremely responsive to experimental circumstances, and even little deviations might result in substantial alterations in the reported results. This sensitivity poses a dual difficulty and opportunity: although it complicates the execution of studies, it also enables RICD to yield exceptionally precise information about solvation settings.
The influence of solvation on resonance-induced coulombic decay (RICD):
The solvation environment is crucial in the process of reverse intersystem crossing dynamics (RICD). The configuration and kinetics of solvent molecules can have an impact on both the effectiveness and outcome of the degradation process. In polar solvents such as water, the powerful interactions between the solvent and solute can cause notable changes in the resonance conditions, which in turn affect the Resonant Intermolecular Coulombic Decay process.
Furthermore, various solvents can result in distinct reactive intermediate chemical dynamics (RICD) pathways. For example, solvents that create powerful hydrogen bonds might stabilize specific states of the solute, resulting in distinct degradation pathways compared to non-polar solvents. Gaining a comprehensive understanding of these consequences is crucial for effectively utilizing RICD in practical contexts.
The fields of chemistry and biology utilize RICD:
RICD is not only a subject of theoretical interest but also has practical uses, especially in areas where a deep understanding of solvation is essential. Solvation is a critical factor in biological processes such as protein folding, enzyme function, and drug interactions. Scientists can acquire valuable knowledge through the use of Resonant Intermolecular Coulombic Decay to investigate these processes, which may result in the development of novel therapeutic approaches or enhanced pharmaceuticals.
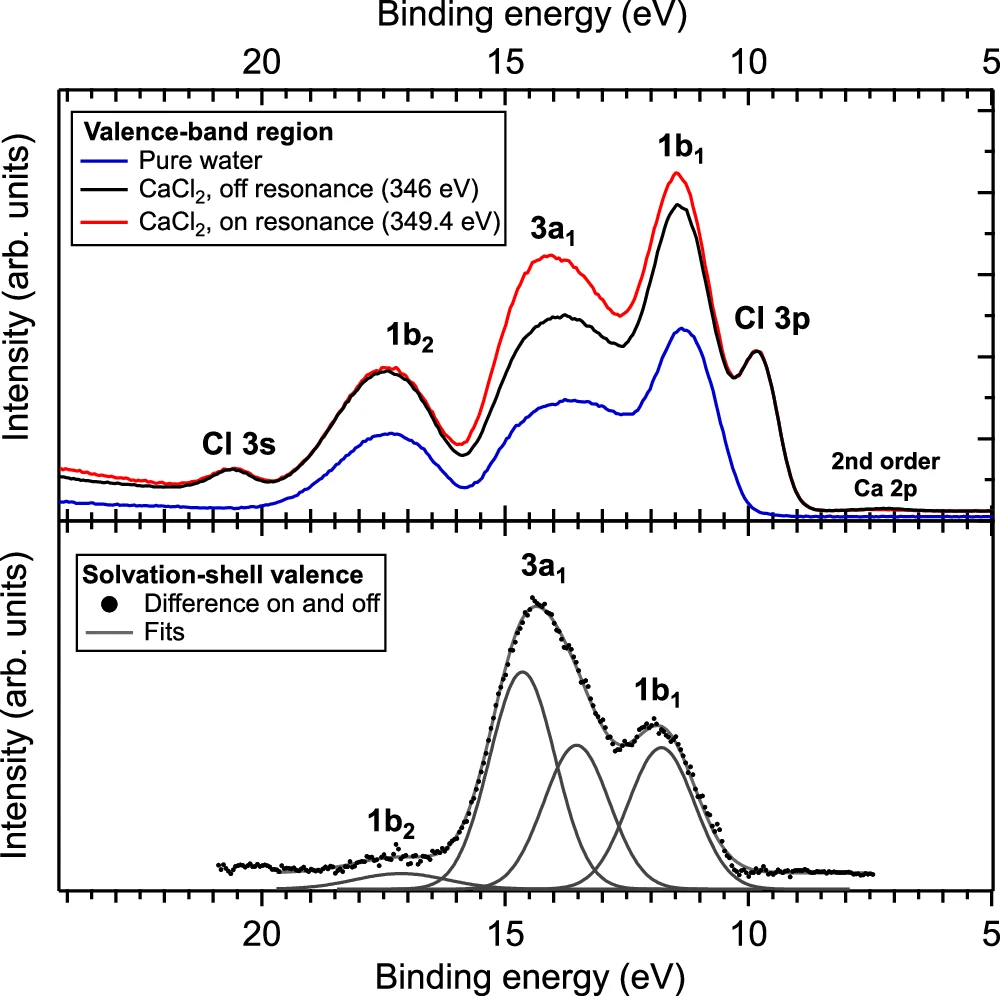
RICD, or Resonant Inelastic X-ray Scattering, is a useful technique in chemistry for investigating catalysis. In catalytic processes, the solvation shell plays a crucial role in determining both the efficiency and selectivity of the reactions. By understanding how solvation affects these processes, chemists may be able to make catalysts that work better or more selectively, which would help the development of more environmentally friendly manufacturing methods. Determination of the valence-band spectrum of water molecules in the solvation shell of Ca2+.
Prospects for Future Research in RICD:
The RICD field is now in its early stages, with numerous promising advancements expected soon. With the ongoing advancement of experimental methodologies, we anticipate an increase in the level of detail and accuracy in studies about solvation shells. Scientists will be able to investigate Resonant Intermolecular Coulombic Decay in real time using emerging technologies like ultrafast lasers and sophisticated detectors, which will provide a dynamic representation of solvation processes.
Furthermore, as computer models become more complex, they will provide more accurate predictions and profound insights, directing future experiments and aiding in the understanding of RICD’s intricacies. Numerous questions in the discipline remain unresolved, necessitating a blend of experimental ingenuity and theoretical progress to tackle them.
Obstacles and Constraints:
RICD research encounters various obstacles, despite its considerable potential. Due to the RICD’s susceptibility to experimental circumstances, meticulous planning and execution of studies are required, typically including costly and intricate equipment. Another thing is that the theoretical frameworks used to explain RICD are still being improved, and there is still a lot of uncertainty about the basic quantum mechanisms at play.
Another constraint arises from the challenge of investigating RICD in intricate systems, such as sizable biomolecules or heterogeneous solvents. These systems introduce additional variables that increase the complexity of both the experiments and the interpretation of outcomes. Successfully addressing these obstacles will be crucial to realizing the complete capabilities of Resonant Intermolecular Coulombic Decay.
In conclusion:
To conclude, resonant intermolecular interactions Coulombic decay is a powerful and new way to study the solvation shell, which is an important but hard-to-understand part of chemical reactions. Scientists may examine the solvation shell and obtain intricate knowledge on the arrangement and movement of solvents using RICD. This has significant implications for various disciplines, including biology and industrial chemistry. Despite some obstacles, the prospects for RICD research appear optimistic, offering the potential to uncover further insights into the captivating realm of solvation.
Commonly Asked Questions (CAQs):
1). The solvation shell in chemistry holds enormous importance due to its significant role in the process of solvation.
The solvation shell is critical because it has a significant impact on the chemical properties and reactivity of solutes in a solvent. This influence extends to various aspects, ranging from enzyme function to ion transport.
2). What are the differences between resonant intermolecular Coulombic decay and normal ICD?
When the excitation energy is the same as the energy difference between states, there is resonant interatomic Coulombic decay (RICD). This makes the decay process stronger than normal interatomic Coulombic decay (ICD).
3). Is it possible to use RICD to analyze biological systems?
Indeed, RICD provides a valuable understanding of phenomena like protein folding and enzyme activity by investigating solvation in biological systems.
4). What are the primary obstacles encountered when studying RICD?
The challenges include RICD’s susceptibility to experimental variables, the complexity of theoretical frameworks, and the obstacles encountered when investigating intricate systems.
5). What potential effects could advancements in RICD research have on future scientific progress?
Progress in research on resonance-induced charge delocalization (RICD) has the potential to result in improved catalysts, novel medicinal approaches, and an enhanced understanding of solvation in chemical and biological systems.
For more chemistry blogs, visit chemistry Master