Table of Contents
Opening statement:
In the pursuit of sustainable and effective energy sources, scientists and researchers have been investigating many approaches, including the extraction of uranium from seawater. Nuclear energy, a significant alternative to traditional fossil fuels, relies on uranium as an essential component. Conventional uranium mining is both expensive and harmful to the environment. As a result, the development of a technique that enables the effective retrieval of uranium from saltwater is a groundbreaking achievement. This article examines the extremely efficient process of enriching uranium via the growth-elution cycle of studtite nanodots.
Understanding the uranium enrichment process:
The process of increasing the concentration of the isotope uranium-235 in natural uranium, which primarily consists of uranium-238, is known as uranium enrichment. We do this to produce fuel for nuclear reactors or to create materials for nuclear weapons.
The process of uranium enrichment enhances the concentration of uranium-235 in uranium. Uranium-238 makes up around 99.3% of natural uranium, while uranium-235 accounts for only 0.7%. To optimize the performance of nuclear reactors and weapons, it is necessary to increase the concentration of uranium-235 through enrichment, as this particular isotope is capable of undergoing fission and releasing energy.
There are conventional techniques for increasing the concentration of uranium:
Techniques such as gaseous diffusion and gas centrifuges have historically accomplished uranium enrichment. Gaseous diffusion is a process that separates uranium isotopes by taking advantage of their different molecular weights. This is a barrier with small holes or pores A gas centrifuge is a device that uses rapid rotation to separate isotopes. Both approaches exhibit considerable energy use, intricate processes, and substantial costs, underscoring the necessity for more efficient and sustainable alternatives. Synthesis and structures.
Seawater Uranium:
Uranium’s presence in seawater:
Seawater contains around 4.5 billion metric tonnes of uranium, significantly more than the Earth’s land-based reserves. If we develop an efficient extraction method, seawater has the potential to serve as an abundant and virtually limitless supply of uranium. Nevertheless, the quantity of uranium in saltwater is exceedingly low, approximately 3.3 parts per billion, which presents a substantial obstacle to extraction.
There are challenges associated with the extraction process of uranium from saltwater:
The primary obstacle to extracting uranium from seawater is its low concentration. Moreover, seawater has a multitude of diverse ions and chemicals that can disrupt the extraction procedure. To achieve optimal efficiency, the extraction material must possess a high degree of selectivity towards uranium. Conventional extraction procedures are not suitable for dealing with these problems, necessitating new methods, such as the use of studtite nanodots.
An Introduction to Studtite Nanodots:
What is the definition of studtite nanodots?
Studtite nanodots are minute particles at the nanoscale that originate from the mineral studtite, specifically a uranium peroxide hydrate. The nanodots possess distinctive chemical characteristics and a large surface area, which enhances their ability to absorb uranium ions from seawater.
Stutite nanodots have distinct characteristics:
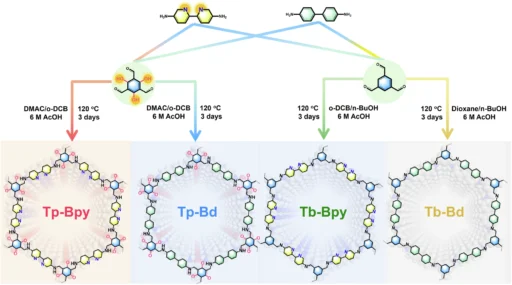
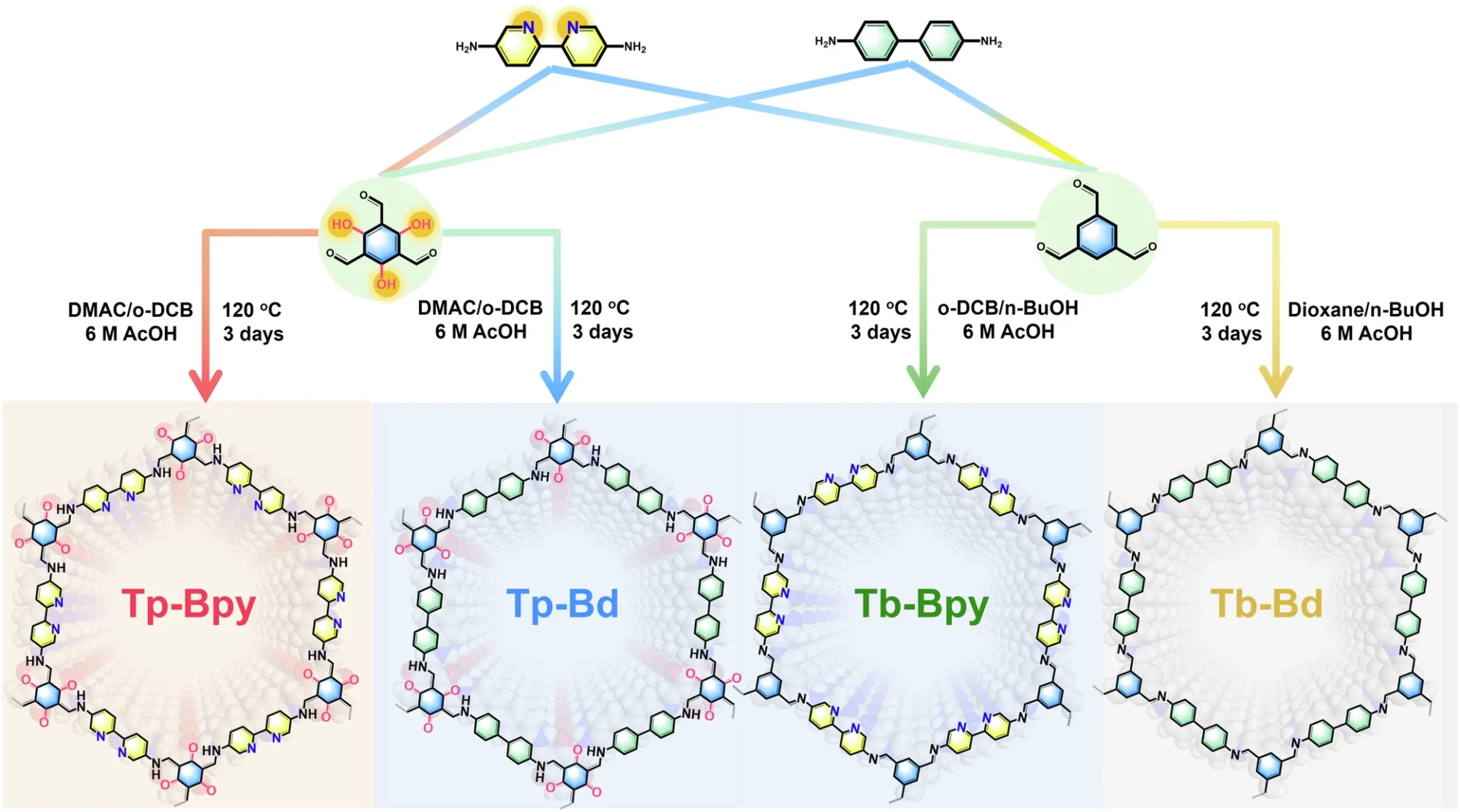
Studtite nanodots exhibit unique characteristics, including a strong preference for uranium ions, resilience in marine environments, and the ability to regenerate and utilize them repeatedly. These attributes make them well-suited for a sustainable and effective method of extracting uranium. Chemical structure and characterization of Tp-Bpy, Tp-Bd, Tb-Bpy and Tb-Bd.
The Growth-Elution Cycle Method is a technique for substance analysis or separation:
Exposition of the growth-elution cycle:
The growth-elution cycle is a novel technique for collecting uranium from saltwater using studtite nanodots. This procedure entails cultivating nanodots, exposing them to seawater to adsorb uranium, and then eluting (releasing) the uranium for retrieval. You can iterate the process numerous times, thereby improving efficiency and sustainability.
The growth-elution cycle consists of several steps:
Growth: We fabricate and apply studtite nanodots to a compatible substrate.
Immersing the substrate containing nanodots in seawater causes the nanodots to attract and bind uranium ions.
Elution: We subject the uranium-containing nanodots to a solution to liberate the uranium that has adsorbed onto them.
Regeneration: To prepare for the next cycle of exposure and removal, we restore the nanodots to their original state.
The Mechanism of Studtite Nanodots in Uranium Extraction:
The application of studtite nanodots enhances uranium extraction:
Studtite nanodots improve the uranium extraction process because of their large surface area and strong chemical attraction to uranium ions. The nanodots’ structure allows for a high concentration of adsorption sites, resulting in their exceptional efficiency in absorbing uranium from seawater.
Benefits compared to conventional extraction methods:
Studtite nanodots have numerous benefits in comparison to conventional techniques. They exhibit greater selectivity for uranium, consume lower amounts of energy, and are less susceptible to the influence of other ions found in seawater. Moreover, the process of regeneration is simpler and more economical, thereby minimizing the overall ecological footprint. The schematic diagram for the reaction process of Tp-Bpy and Tb-Bpy.
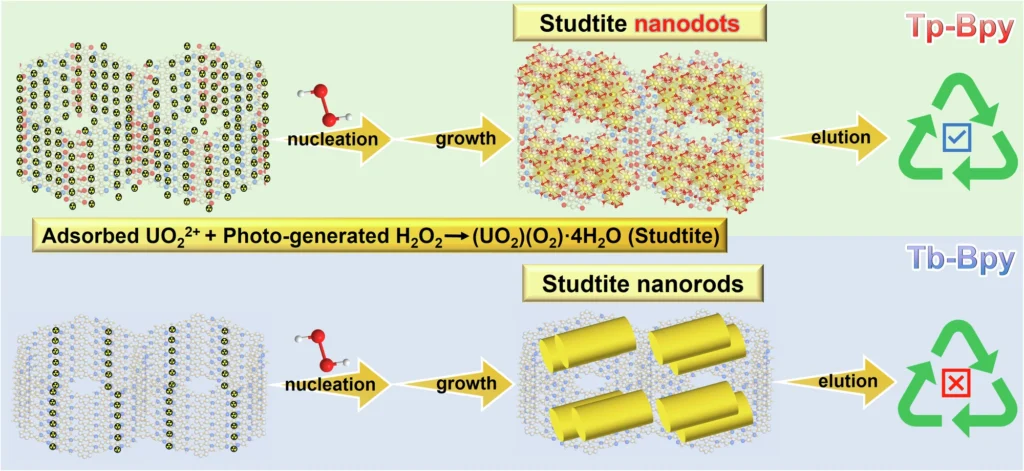
The efficacy of the growth-elution cycle:
The study focuses on the comparative efficacy of alternative methods:
Compared to conventional approaches, the growth-elution cycle with studtite nanodots exhibits markedly superior efficiency. Research suggests that this approach can reach uranium recovery rates over 90%, whereas conventional approaches have challenges achieving lower recovery rates due to competition from other ions and higher operational expenses.
Contributing factors to achieving high efficiency:
Multiple variables contribute to this method’s exceptional efficiency:
Significant surface area: The nanodots provide a large surface area for uranium adsorption.
Selective binding: Studtite nanodots have chemical properties that guarantee exclusive uranium ion binding.
Regeneration capacity: The ability to restore nanodots for many cycles reduces costs and eliminates waste.
Case Studies and Research Findings:
Overview of recent scientific research investigations:
Recent research has shown encouraging results regarding the use of studtite nanodots in the extraction of uranium from seawater. Extensive laboratory studies and field testing have shown that the recovery rates are high and the system can operate efficiently in different maritime environments.
The approach’s efficacy is demonstrated through illustrative case studies:
In a significant case study, researchers introduced studtite nanodots into coastal waters and reported a 90% uranium recovery rate. This study emphasized the feasibility and expandability of the growth-elution cycle, demonstrating its potential for broad implementation. Pore structure and photoelectrochemical tests for Tp-Bpy, Tp-Bd, Tb-Bpy and Tb-Bd.
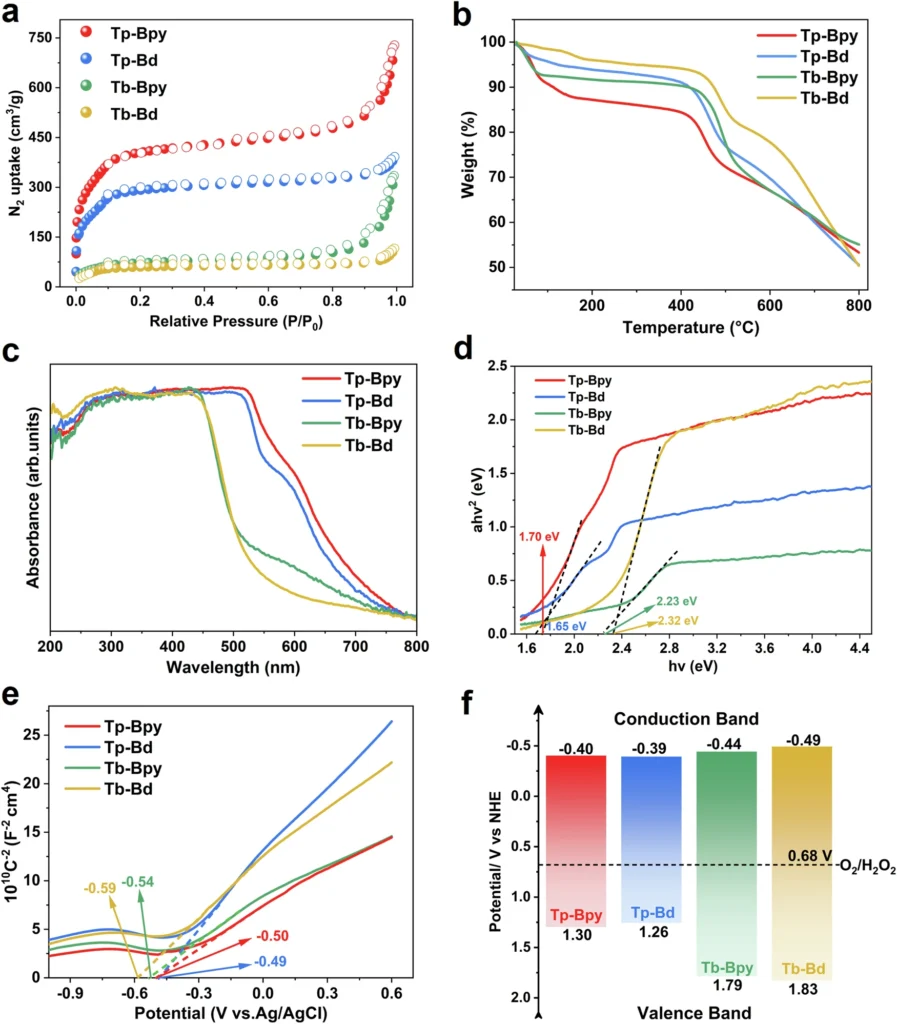
Ecological Consequences:
The environmental benefits of using studtite nanodots are significant:
Using studtite nanodots for uranium extraction has notable ecological advantages. Unlike conventional mining practices that result in land degradation and the destruction of habitats, this approach operates in marine areas with minimal ecological disturbance. Moreover, the decreased energy usage results in a reduction of carbon emissions linked to the process of extracting uranium.
Possible environmental concerns:
Although there are advantages, it is crucial to acknowledge and resolve any potential ecological issues. The potential ramifications of introducing nanomaterials into the ocean over an extended period remain incompletely comprehended, necessitating additional research to ascertain that this approach does not inflict any detrimental effects on marine ecosystems. To effectively manage these risks, regulatory frameworks must be implemented.
Economic Viability
Economic Viability The study focuses on the cost analysis of using studtite nanodots for uranium extraction.
Preliminary cost estimates indicate that the growth-elution cycle using studtite nanodots has the potential to be economically viable compared to conventional uranium mining. The decreased energy demands and the capacity to regenerate nanodots result in reduced operational expenses. We anticipate further cost reductions as the technology expands, thereby enhancing its economic feasibility.
Contrast with traditional approaches:
The growth-elution cycle is more cost-effective than conventional approaches for various reasons, including:
Reduced energy consumption: conventional techniques, such as gas centrifuges, exhibit a high level of energy intensity.
Decreased material expenses: The capacity to regenerate studtite nanodots diminishes the desire for constant material input.
Operational simplicity: The process is characterized by reduced complexity, necessitating a smaller number of specialized facilities and equipment.
Addressing technical challenges and providing solutions:
The implementation of the growth-elution cycle presents challenges from a technical standpoint.
The implementation of the growth-elution cycle poses a variety of technological challenges:
Stability of nanodots: Ensuring the long-term stability of studtite nanodots in seawater is of utmost importance.
Enhancement of the elution process: Achieving the efficient liberation of uranium from the nanodots without compromising their structural integrity is a crucial technical challenge.
Scalability: Achieving industrial-scale implementation necessitates substantial research and development efforts.
Potential remedies and current investigations:
Continued research efforts aim to address these challenges by:
Enhancing nanodot stability: We are developing coatings or changes to improve the stability of studtite nanodots in marine settings.
Improving elution techniques: Enhancing elution methods involves conducting trials with various elution agents and techniques to enhance the retrieval of uranium while ensuring the preservation of nanodots.
Scaling strategies: There are several methods for increasing the size or capacity of a system or process. We can develop modular systems that can operate at various scales, from small-scale experiments to extensive industrial activities. Photocatalytic performance of Tp-Bpy, Tp-Bd, Tb-Bpy, and Tb-Bd for uranyl extraction and H2O2 production.
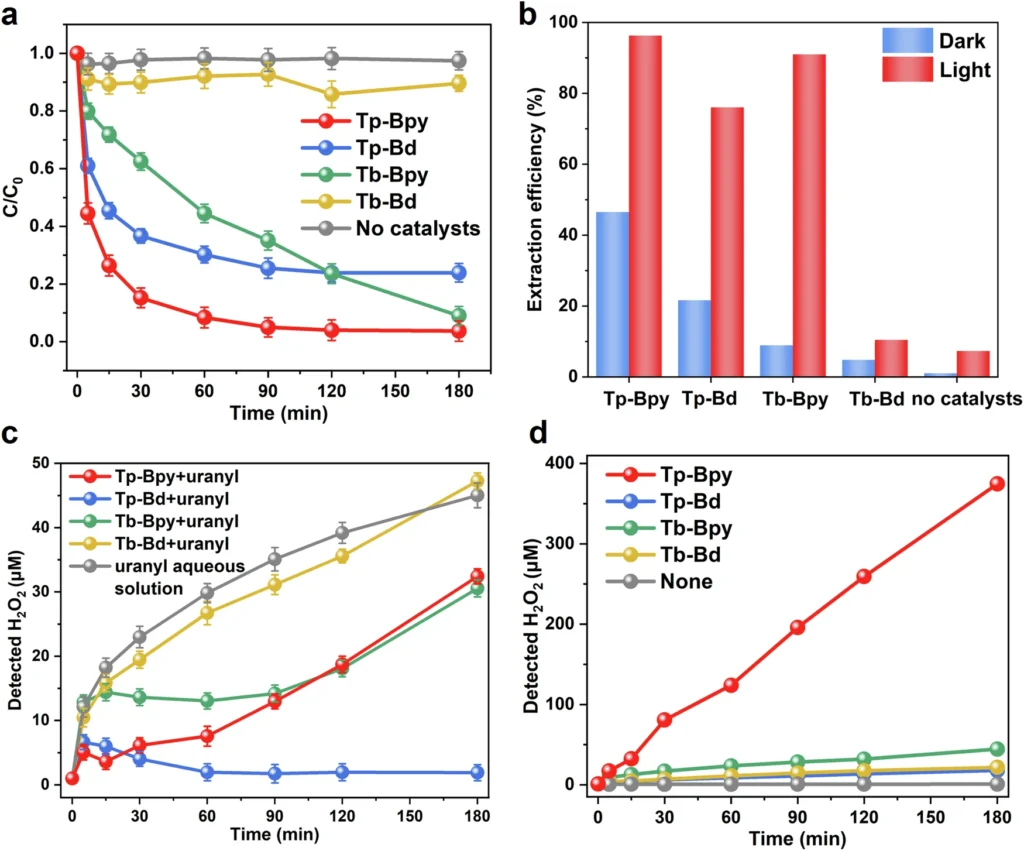
Prospects for the future:
Possible developments in technology:
The prospects for utilizing studtite nanodots in uranium extraction appear highly favorable, as there are numerous potential breakthroughs on the horizon.
Hybrid nanomaterials: To improve efficiency and selectivity, hybrid nanomaterials combine studtite nanodots with other substances.
Improved regeneration methods: We are enhancing regeneration methods by advancing the development of regeneration strategies that are both more efficient and economically viable.
Large-scale deployment systems: We are developing expansive deployment systems by constructing adaptable and resilient systems for extensive use in marine settings.
The approach has potential future applications:
Apart from uranium extraction, we can also use the growth-elution cycle and nanomaterials to extract other valuable elements from saltwater, such as lithium and rare earth metals. This presents new opportunities for recovering resources from the ocean.
We must consider policy and regulatory factors:
Existing legislation related to the extraction of uranium from seawater:
Currently, laws primarily cater to land-based uranium mining. To ensure environmental protection and sustainable practices during the extraction of uranium from seawater using studtite nanodots, new regulatory frameworks will be required.
Policy changes needed to support new methods:
Reforms are required to facilitate the implementation of innovative approaches:
Environmental safeguards: Implementing environmental protections involves creating specific protocols to systematically monitor and minimize the ecological consequences of using nanodots in marine settings.
Research funding: enhancing financial resources allocated to research and development to optimize technological advancements and tackle technical obstacles.
Incentives for innovation: We are encouraging innovation by offering incentives to corporations and research organizations to create and apply environmentally friendly techniques for extracting uranium.
Worldwide Influence:
The potential impact of widespread adoption on the energy landscape is worth considering.
The worldwide energy environment could undergo substantial changes due to the extensive use of studtite nanodots for uranium extraction. It would establish a dependable and enduring supply of uranium, diminishing dependence on land-based mining and bolstering energy security. This approach could additionally facilitate the shift towards low-carbon energy sources, bolstering worldwide endeavors to mitigate climate change.
Potential benefits for different nations:
Nations with long coastlines and the ability to access saltwater have the potential to emerge as significant participants in uranium extraction, thereby stimulating their economies and reinforcing their energy self-sufficiency. This approach has the potential to foster global collaboration in the development and dissemination of sustainable energy technology.
In conclusion:
The enrichment of uranium from seawater using the studtite nanodot growth-elution cycle is an extremely efficient method, indicating a major advancement in resource extraction technology. This approach provides multiple benefits, such as exceptional effectiveness, ecological sustainability, and economic feasibility. Continued study and development of this technology have the potential to revolutionize the worldwide energy sector by offering a sustainable answer to the increasing need for nuclear fuel. This approach’s extensive use has the potential to result in a more environmentally friendly and reliable energy future by implementing appropriate rules and regulatory frameworks.
Frequently Asked Questions:
1). What is the mechanism behind the functioning of studtite nanodots in the process of uranium extraction?
Studtite nanodots have a strong affinity for uranium ions, effectively adsorbing them from seawater in a selective manner. Due to their distinctive chemical composition and large surface area, they exhibit exceptional efficacy in collecting uranium.
2). What factors contribute to the efficiency of the growth-elution cycle?
The growth-elution cycle takes advantage of studtite nanodots’ extensive surface area and specific binding characteristics, allowing for repeated use and efficient retrieval with minimal energy consumption.
3). Are there any potential environmental hazards related to this approach?
Although the approach provides substantial ecological advantages, there is a possibility of releasing nanomaterials into marine environments, which poses potential hazards. Additional research is required to comprehensively comprehend and address these dangers.
4). What is the cost comparison between this method and traditional methods?
This approach has the potential to be more cost-effective because it uses less energy and reduces operational costs. The capacity to regenerate nanodots significantly improves economic feasibility, especially as the technology expands.
5). What are the future possibilities for extracting uranium from seawater?
The future outlook is optimistic, as improvements in material science and process engineering can expand the uses and enhance the efficiency of extracting resources from saltwater. We can also use this approach to extract other useful components, thereby creating new opportunities for sustainable resource retrieval.
For more chemistry blogs, visit chemistry Master