Table of Contents
The opening statement of Suzuki Miyaura Coupling:
The Suzuki Miyaura coupling reaction is a fundamental process in organic synthesis, highly esteemed for its efficacy in creating carbon-carbon bonds. Experts in chemistry have completely changed how they think about making complex molecules since this reaction was first used to make biaryls and other polyaromatic compounds. However, just like any other chemical reaction, the specific conditions under which Suzuki-Miyaura coupling occurs can significantly influence its outcome. Lately, there has been an increasing fascination with utilizing arylthianthrenium tetrafluoroborate salts under acidic circumstances to improve the efficiency and range of the reaction. This article explores the intricacies of this strategy, analyzing the fundamental mechanisms, benefits, difficulties, and possible uses.
Historical Context of the Suzuki Miyaura coupling :
Akira Suzuki and his research team first identified the Suzuki Miyaura coupling in the late 1970s, and it quickly became a crucial technique for creating carbon-carbon bonds. Initially, the reaction primarily used aryl halides and boronic acids, but it has gradually expanded to include a variety of substrates and conditions. Conventional Suzuki coupling reactions frequently necessitate neutral or somewhat basic conditions. Despite their success, these conditions could limit the range of available substrates. As a result, researchers began investigating novel reagents and circumstances, such as arylthianthrenium tetrafluoroborate salts and acidic environments.
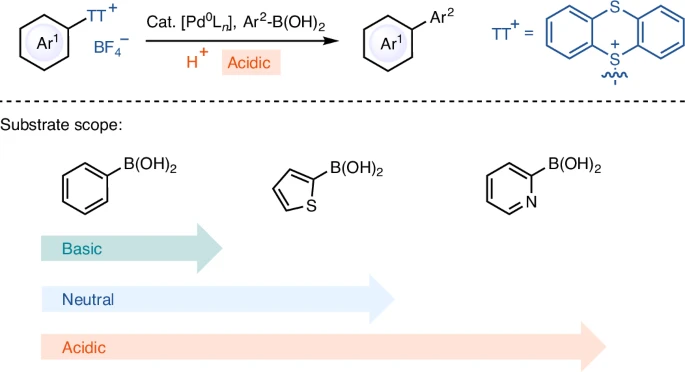
Chemical structure of arylthianthrenium tetrafluoroborate salts:
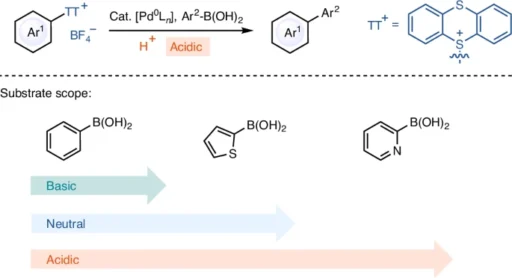
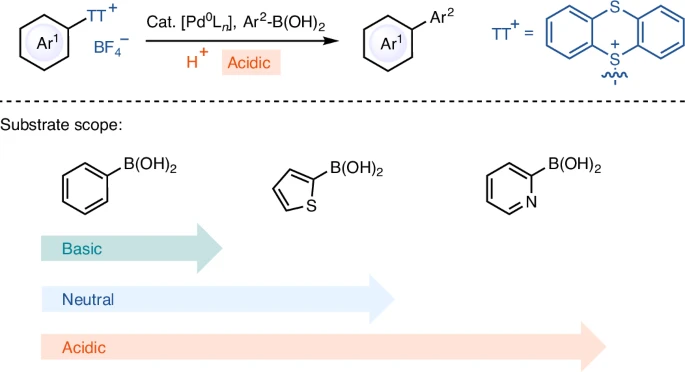
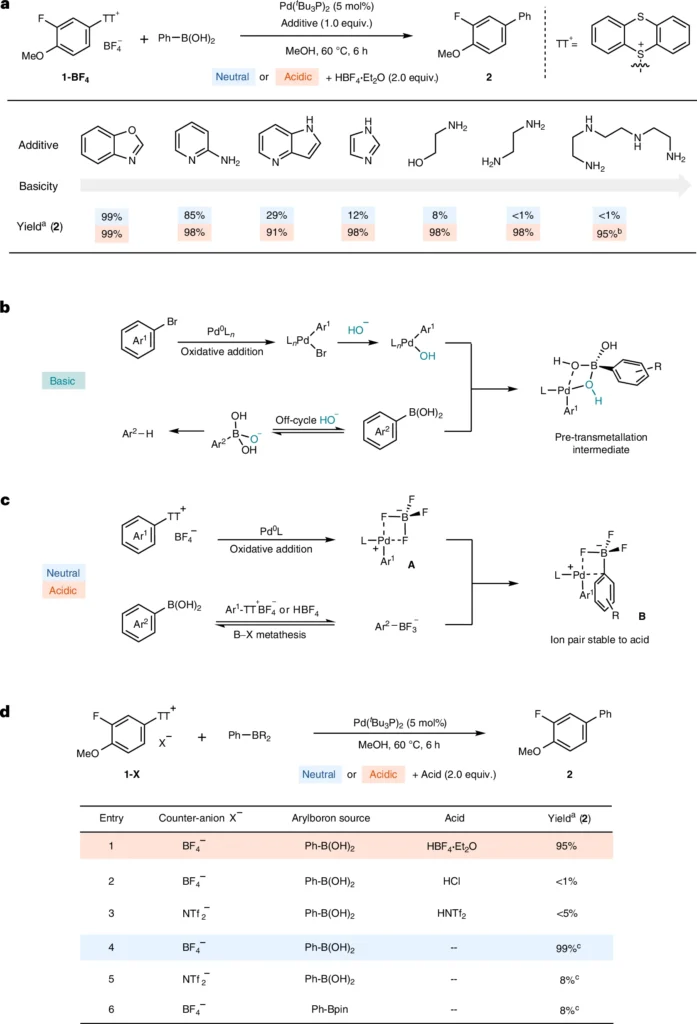
Arylthianthrenium tetrafluoroborate salts are a novel type of coupling partner, characterized by their unusual chemical structure. An aryl group connects to a thianthrene core and combines with a tetrafluoroborate anion to form the salts. The thianthrene moiety exhibits remarkable stability and reactivity, especially in acidic environments. Compared to other aryl halides, arylthianthrenium salts are more reactive, which means they can couple with less reactive substances more efficiently.
The Suzuki Miyaura coupling reaction has a mechanism:
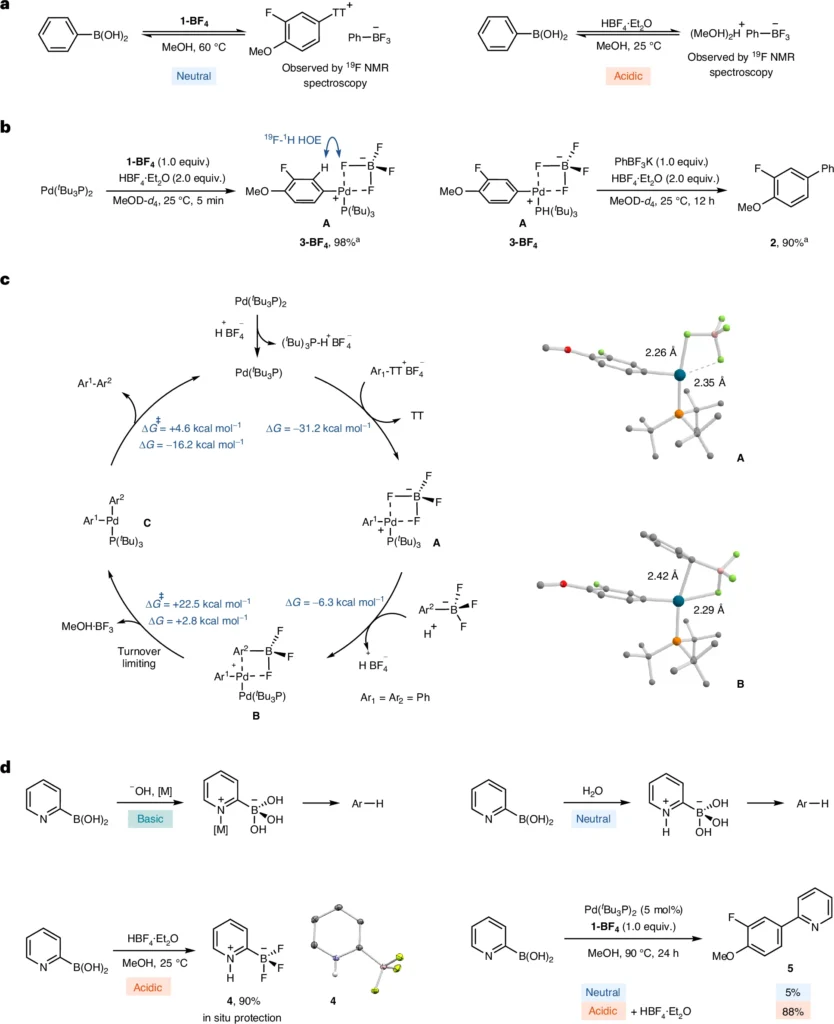
The Suzuki Miyaura coupling mechanism is a widely recognized process that consists of three essential steps: oxidative addition, transmetalation, and reductive elimination. During the oxidative addition process, a palladium(0) catalyst reacts with the arylthianthrenium salt, forming a palladium(II) complex. Subsequently, the boronic acid partner assists in transmetalation, transferring the aryl group from the thianthrene core to the palladium complex. Ultimately, reductive elimination liberates the interconnected product and restores the palladium(0) catalyst.
Under acidic conditions, the reaction process can display significant variations. Acid can increase the electrophilicity of the palladium complex, leading to accelerated oxidative addition. In addition, acidic conditions can enhance the stability of the intermediates, inhibiting side reactions and resulting in increased yields. Suzuki Miyaura coupling.
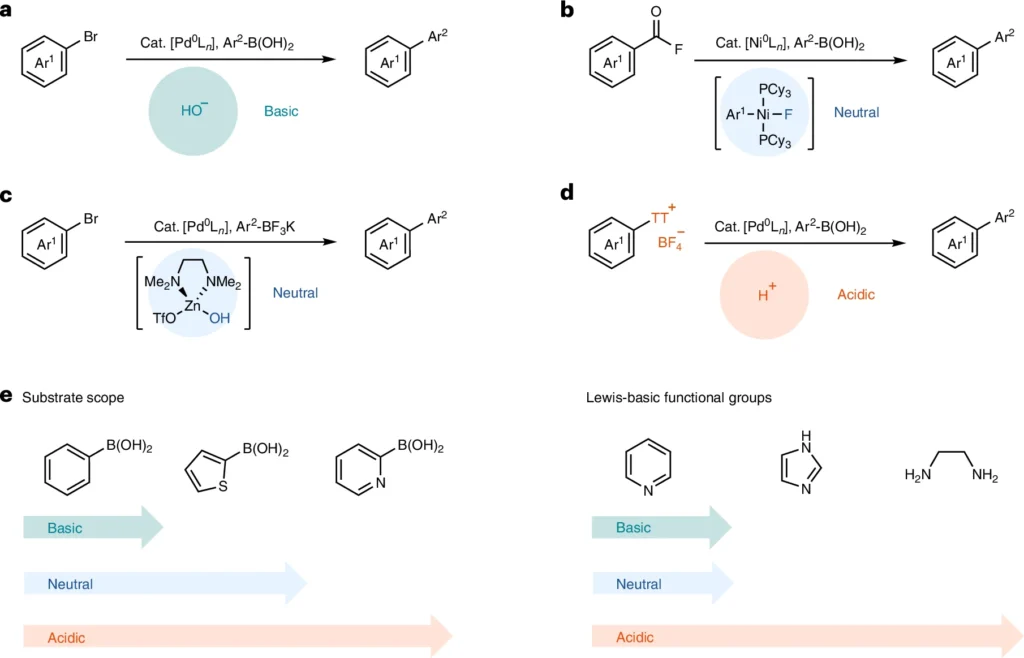
Mechanistic analysis.
Acidic conditions play a crucial role in the coupling process:
Acidic conditions significantly influence the efficacy and selectivity of the Suzuki Miyaura coupling when utilizing arylthianthrenium tetrafluoroborate salts. The acidity can impact multiple facets of the process, ranging from the platinum catalyst’s reactivity to the intermediates’ stability. This reaction commonly employs Trifluoroacetic acid (TFA), sulfuric acid, and hydrochloric acid. These acids vary in their ability to donate protons and stabilize the reaction. Given these circumstances, the reaction frequently advances more efficiently, with a reduced incidence of byproducts, making it an appealing choice for complex substrates.
Benefits of Utilizing Arylthianthrenium Tetrafluoroborate Salts in Suzuki Miyaura Coupling:
An important benefit of employing arylthianthrenium salts in Suzuki Miyaura coupling is their heightened reactivity. These salts have high efficacy in facilitating coupling reactions that are slow or unresponsive when using conventional aryl halides. Furthermore, they provide enhanced stability across many circumstances, including acidic environments, which is essential for some synthetic applications. Additionally, their wide range of substrates makes them highly adaptable for use in the synthesis of intricate chemical compounds. Suzuki Miyaura coupling reaction of arylthianthrenium salts.
There are obstacles and factors to consider:
Although arylthianthrenium tetrafluoroborate salts have benefits, there are difficulties when employing them in acidic environments. One potential concern is the occurrence of side reactions, particularly the protonation of the palladium complex, which could lead to the catalyst’s deactivation. Moreover, the meticulous preparation and management of these salts necessitate thoughtful deliberation, as they can be susceptible to moisture and air. Scaling up for industrial uses presents difficulties, especially in ensuring consistent reactions and controlling expenses.
Enhancement of Reaction Conditions:
To enhance the efficiency of the Suzuki Miyaura Coupling process using arylthianthrenium salts, it is necessary to carefully adjust multiple parameters. The temperature and reaction time are crucial factors, as greater temperatures typically result in accelerated responses, albeit with an elevated likelihood of adverse reactions. Proper catalyst loading and ligand selection are critical factors that can have a significant impact on both the efficiency and specificity of the reaction. The solvent choice is critical, as polar solvents typically offer improved solubility for the reactants and intermediates, thereby increasing the reaction’s efficiency.
Methodology:
To carry out a Suzuki Miyaura Coupling reaction utilizing arylthianthrenium tetrafluoroborate salts in an acidic environment, one can use the following standard procedure:
Procedure: Place the arylthianthrenium tetrafluoroborate salt, boronic acid, palladium catalyst (such as Pd(PPh3)4), and the selected acid (such as TFA) into a dry reaction jar.
Solvent Addition: Introduce an appropriate solvent, such as acetonitrile or DMF.
Procedure: Apply heat to the mixture until it reaches the needed temperature, which is usually within the range of 60-100 °C. Stir the mixture for the necessary duration, often lasting between 6 and 24 hours.
Procedure: After completing the workup, let the reaction cool down before adding water to neutralize it. Using an organic solvent such as ethyl acetate, retrieve the product.
Purification: Refine the raw product using either column chromatography or recrystallization.
Applications in Organic Synthesis:
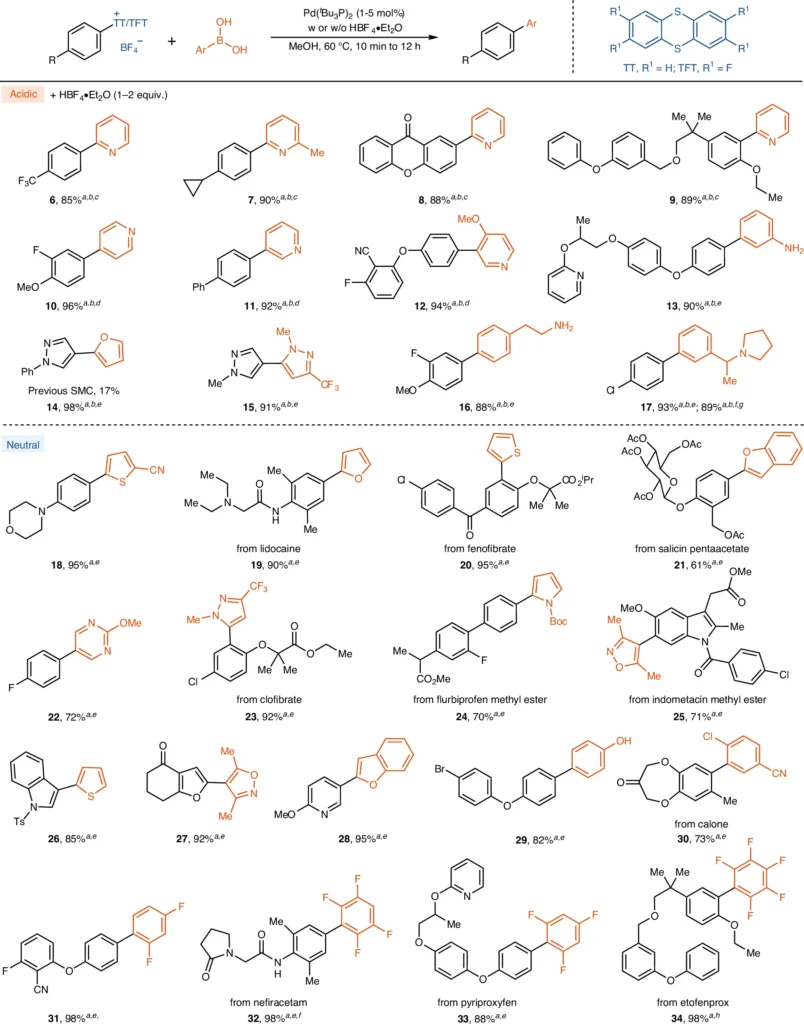
The synthesis of biaryls extensively uses arylthianthrenium salts in Suzuki–Miyaura Coupling reactions under acidic conditions. Biaryls are crucial building blocks in the production of many medicines, agrochemicals, and materials science applications. These reactions are very valuable for generating intricate polyaromatic structures, which can be difficult to synthesize using conventional methods. The method’s resilience and adaptability make it a powerful instrument in both scholarly investigation and industry implementations.
The latest progress and scientific breakthroughs:
Current studies have concentrated on enhancing the effectiveness of Suzuki Miyaura Coupling by creating novel palladium catalysts and investigating alternate acidic environments. Advancements in ligand design have resulted in catalysts that exhibit higher levels of activity and selectivity, even when faced with difficult conditions. Moreover, the investigation of novel acids and reaction media has expanded the potential for combining reactions with substrates that were previously incompatible. Substrate scope for Suzuki Miyaura coupling reaction of arylthianthrenium salts.
Examining specific instances or examples:
A number of case studies demonstrate the effectiveness of using arylthianthrenium salts in the Suzuki Miyaura Coupling process. An example of this technology’s applicability is the effective synthesis of complex biaryls, which are critical intermediates in drug production. Comparative investigations have demonstrated that these salts have superior performance compared to conventional aryl halides in terms of both yield and reaction time, especially under acidic conditions.
We must consider environmental and safety factors:
Like other chemical processes, safety and environmental effects are critical considerations. Acidic environments necessitate meticulous management of both the acids and the resultant waste. The concepts of green chemistry advocate for the minimization of harmful solvents and waste reduction whenever possible. In addition, it is crucial to have adequate ventilation and use personal protection equipment (PPE) when handling reactive salts and acids.
In conclusion:
A very useful and flexible way to make chemicals is to use the Suzuki Miyaura Coupling of arylthianthrenium tetrafluoroborate salts with acid. The increased reactivity and durability of these salts, combined with the advantages of acidic conditions, make this approach a valuable addition to the repertoire of synthetic chemists. As scientific research progresses, we anticipate additional advancements that will broaden the range and effectiveness of this process, presenting new opportunities in the creation of intricate compounds.
Frequently Asked Questions:
1). What distinguishes Suzuki Miyaura Coupling from other methods?
The Suzuki-Miyaura coupling is notable for its high efficiency in forming carbon-carbon bonds, particularly in the synthesis of biaryls, which play a critical role in medicine and materials science.
2). What is the purpose of using arylthianthrenium salts in this reaction?
People use arylthianthrenium salts because they are more reactive and stable in acidic environments. This makes them perfect for difficult coupling processes.
3). What effect do acidic conditions have on the reaction?
When conditions are acidic, the electrophilic nature of the palladium catalyst gets stronger. This makes reaction intermediates more stable and reduces side reactions, which leads to higher yields.
4). What are the primary obstacles in this chemical reaction?
Secondary reactions, managing delicate chemicals, and fine-tuning conditions for large-scale commercial use are all challenges.
5). Is this method applicable on an industrial scale?
Achieving consistency and efficiency on a wider scale demands meticulous optimization of reaction conditions and handling techniques.
For more chemistry blogs, visit chemistry Master