Table of Contents
Preface:
Organic synthesis recognizes palladium (Pd)-catalyzed reactions for their exceptional efficiency and adaptability, establishing them as fundamental in contemporary chemistry. Traditional homogeneous palladium catalysts frequently encounter difficulties, including elevated costs, poor reusability, and challenges in separating from reaction mixtures. This is the role of nanoparticle-based catalysts. Chemists came up with a creative solution by attaching palladium to triazine dendrimers and supporting them with magnetic nanoparticles. This makes the catalyst work better and solves important problems like how to recover the catalyst and how to keep it working.
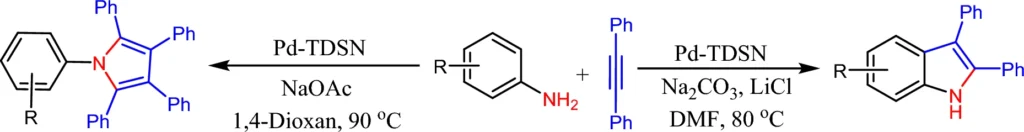
This article looks into how palladium-immobilized triazine dendrimers on magnetic nanoparticles can be used as a reusable microreactor. It focuses on making 2-3-diphenylindoles and pentaphenylpyrrole derivatives in a solvent-dependent way. Scheme 1: Synthesis of 2,3-diphenylindoles and pentaphenyl pyrroles catalyzed by Pd-TDSN.
What are triazine dendrimers?
Triazine dendrimers are extensively branching, tree-like structures distinguished by their recurring triazine units. These synthetic macromolecules have an extensive surface area and adjustable chemical characteristics, rendering them optimal for catalysis. In catalytic applications, triazine dendrimers are better hosts for metal immobilization because they make it easier for the metal to interact with substrates.
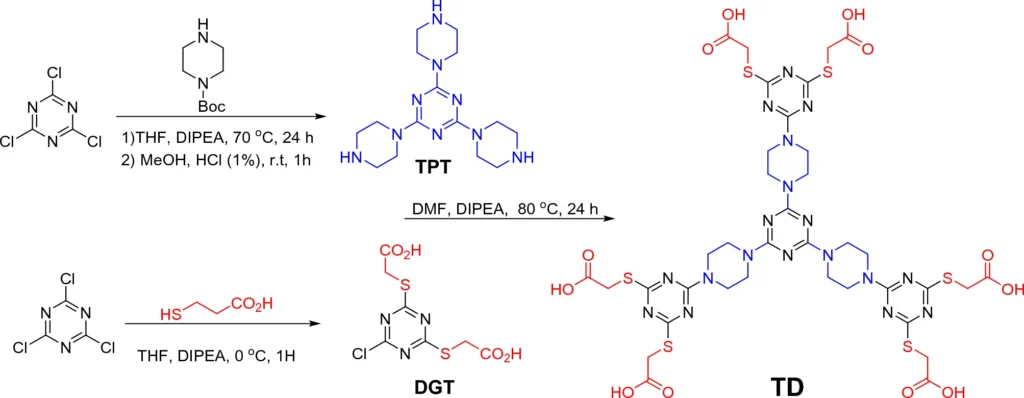
Their multifunctional architecture allows for improved regulation of the reaction environment, making them efficient at immobilizing palladium. Triazine dendrimers are helpful because they keep palladium stable and stop it from leaching, which happens a lot with homogeneous catalysts. Scheme 2: Synthesis of triazine dendrimer through a multistep convergent approach.
Magnetic Nanoparticles in Catalysis:
Magnetic nanoparticles (MNPs) have transformed catalysis by providing a convenient approach for catalyst recovery. These nanoparticles, which are generally made of iron oxide, have magnetic properties that enable the catalyst to be separated from the reaction mixture using an external magnet. This minimizes catalyst attrition, decreases purifying procedures, and enhances catalyst reusability.
The application of MNPs in catalysis presents numerous benefits:
Ease of Recovery: The magnetic characteristics enable the catalyst to be easily extracted following the reaction.
Enhanced Surface Area: MNPs provide a large surface area for palladium immobilization.
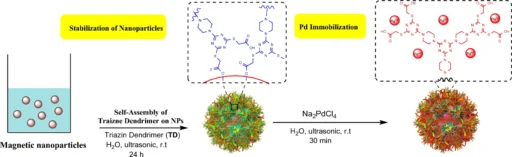
Enhanced Stability: MNP immobilization improves the catalyst’s overall stability. Schematic illustration of stabilization of magnetic nanoparticles by a self-assembling approach and immobilization of palladium ions.
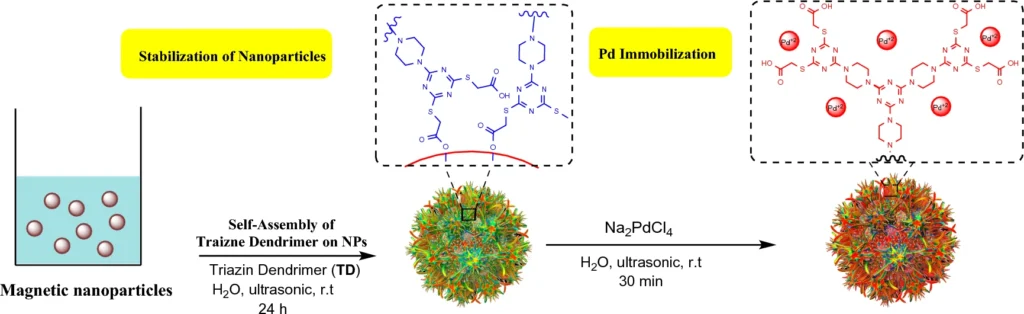
Immobilization of Palladium on Magnetic Nanoparticles:
A multi-step procedure is required to immobilize palladium on magnetic nanoparticles with a triazine dendrimer shell. We first synthesize the triazine dendrimer and then functionalize it to attach palladium ions. We subsequently affix the dendrimers to magnetic nanoparticles, resulting in a stable and highly efficient catalytic system.
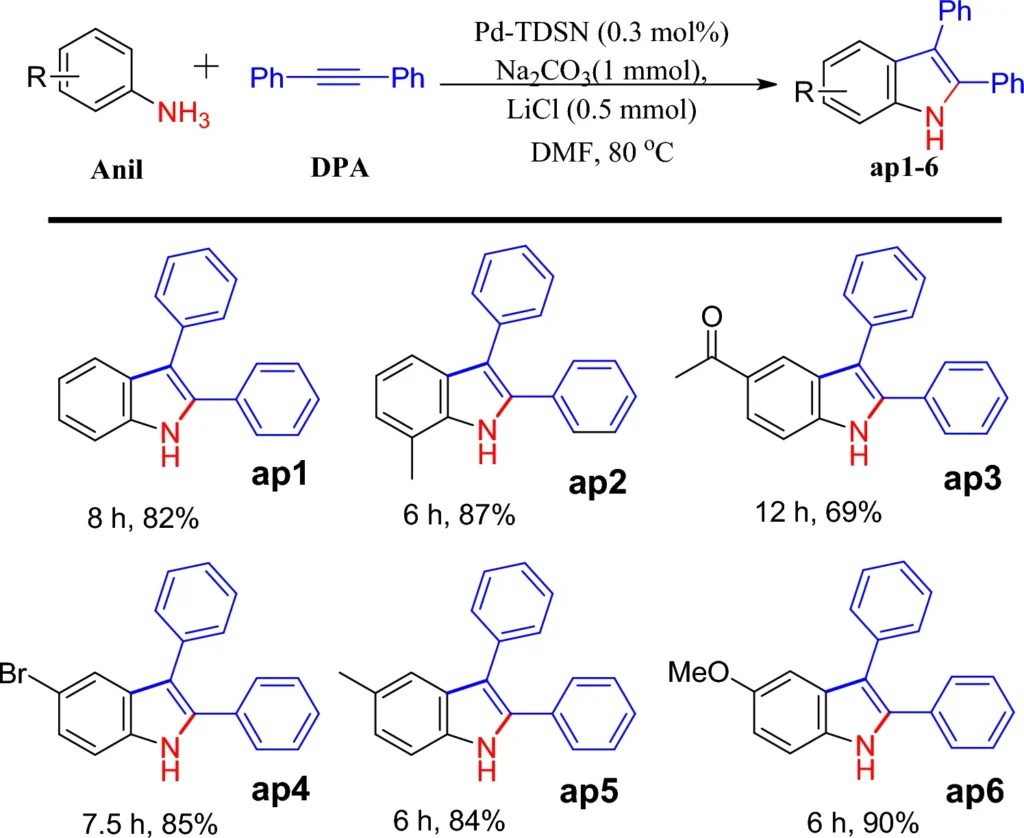
Palladium is particularly suitable for this function because of its established catalytic properties in C-C coupling processes. The immobilized palladium maintains its catalytic efficacy while taking advantage of the dendrimer-MNP combination’s increased surface area and reusability. Scheme 3: Pd-TDSN-catalyzed synthesis of 2,3-diphenylindole.
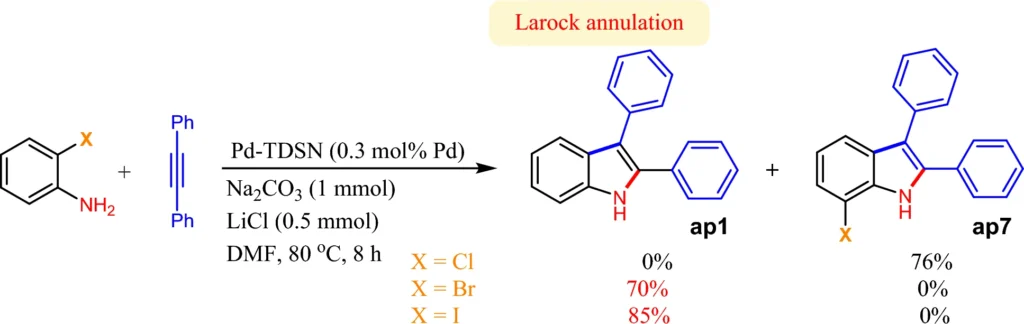
Scheme 4: Larock indole synthesis from 2-haloanilines in the presence of Pd-TDSN.
Synthesis Strategy Dependent on Solvent:
The choice of solvent is critical in determining reaction efficiency, selectivity, and yield. The selection of solvent significantly affects product production in palladium-immobilized triazine dendrimer catalysts. Some solvents can stabilize chemical intermediates, while others may enhance reaction speeds.
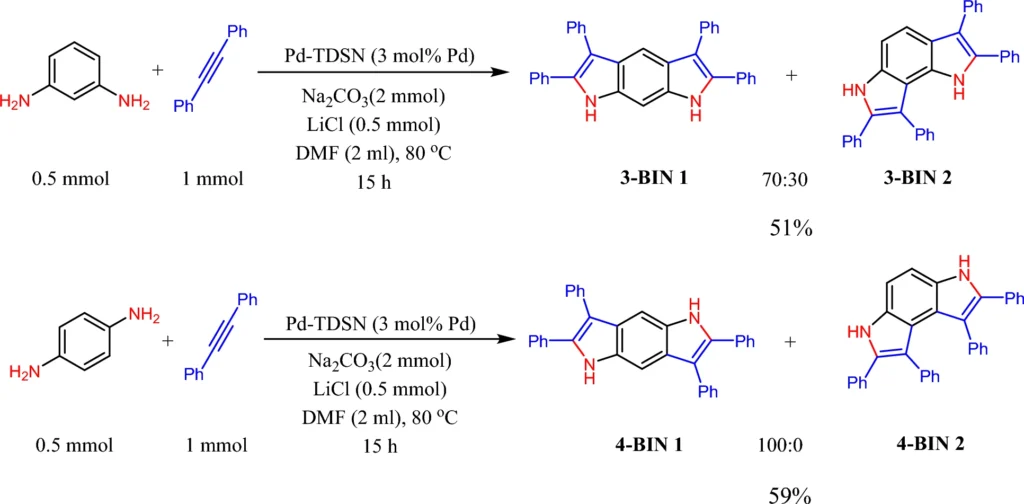
Solvent-dependent synthesis methodologies customize the solvent to enhance specific processes. We may select polar solvents to enhance ionic interactions and employ non-polar solvents to promote hydrophobic substrates. Scheme 5: Pd-TDSN-catalyzed synthesis of bis-indoles.
Synthesis of 2-3-diphenylindoles and pentaphenylpyrrole:
2-3-diphenylindoles and pentaphenylpyrrole are chemical molecules used in various applications, including pharmacology and materials research. The palladium-immobilized catalyst makes it easier for halogenated benzenes and diaryl amines to react with each other, which creates 2-3-diphenylindoles and pentaphenylpyrrole. The solvent employed in this process can affect both the rate and the yield, with polar aprotic solvents frequently preferred.
The catalytic process works very well, and the palladium-based system makes it possible to be very selective, producing 2-3-diphenylindoles and pentaphenylpyrrole with almost no byproducts.
Synthesis of Pentaphenylpyrrole Derivatives:
Pentaphenylpyrrole derivatives are significant substances in organic synthesis, recognized for their structural intricacy and applicability in diverse uses. The synthesis of pentaphenylpyrroles entails the coupling of phenylacetylene derivatives. The palladium-immobilized dendrimer catalyst provides a stable environment for this reaction, and the solvent selection is critical for achieving high yields.
Polar solvents promote pentaphenylpyrrole synthesis, facilitating effective electron transport throughout the process. Scheme 6: Pd-TDSN-catalyzed synthesis of pentaarylpyrroles.
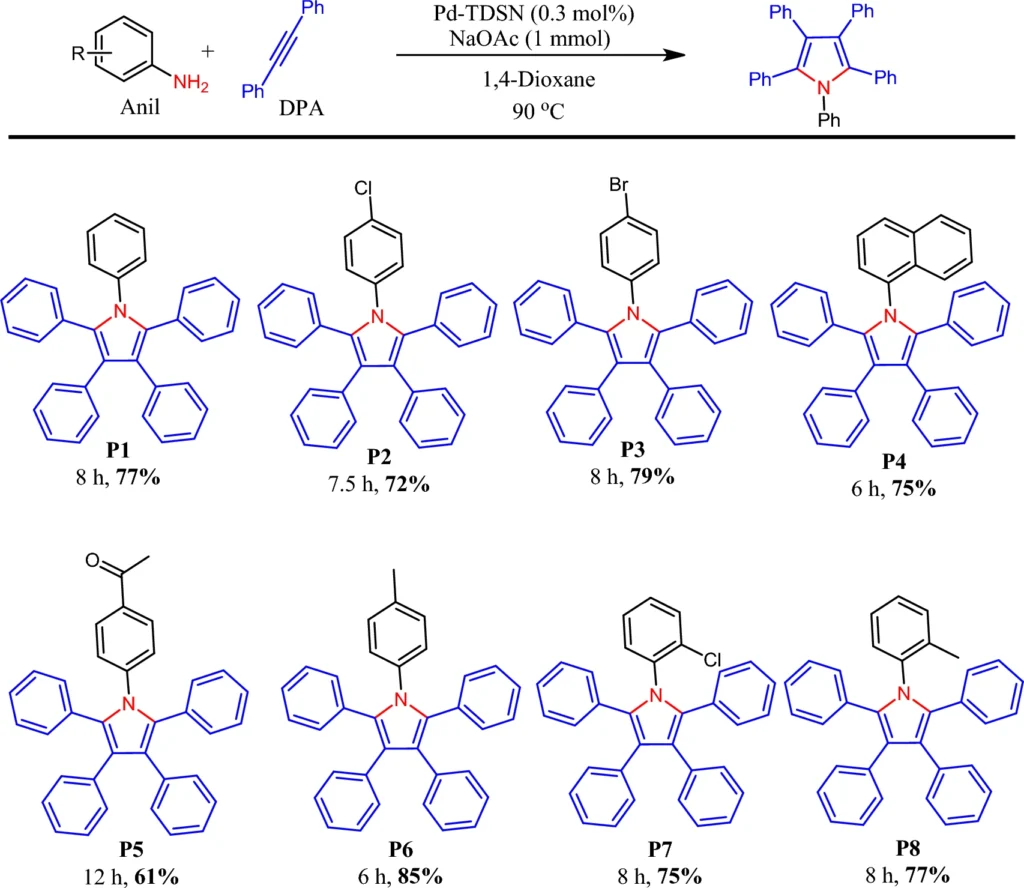
Microreactors: Design and Functionality
A microreactor is a system in which catalytic reactions occur in a meticulously regulated, miniaturized environment. The palladium-immobilized triazine dendrimer on magnetic nanoparticles acts as a microreactor by providing a limited environment for reactants to interact with the palladium catalyst. This configuration enhances reaction rates, boosts efficiency, and facilitates the exact regulation of reaction conditions.
Reusability of the Palladium-Immobilized Catalyst:
The primary benefit of this system is its reusability. After every reaction cycle, a magnet can retrieve the catalyst, purify it, and reuse it with minimal activity loss. According to research, the palladium-immobilized triazine dendrimer on magnetic nanoparticles keeps working well as a catalyst over and over again, making it cost-effective and good for the environment. Computed Gibbs free energy changes of formation P1 and ap1catalyzed by Pd-TDSN.
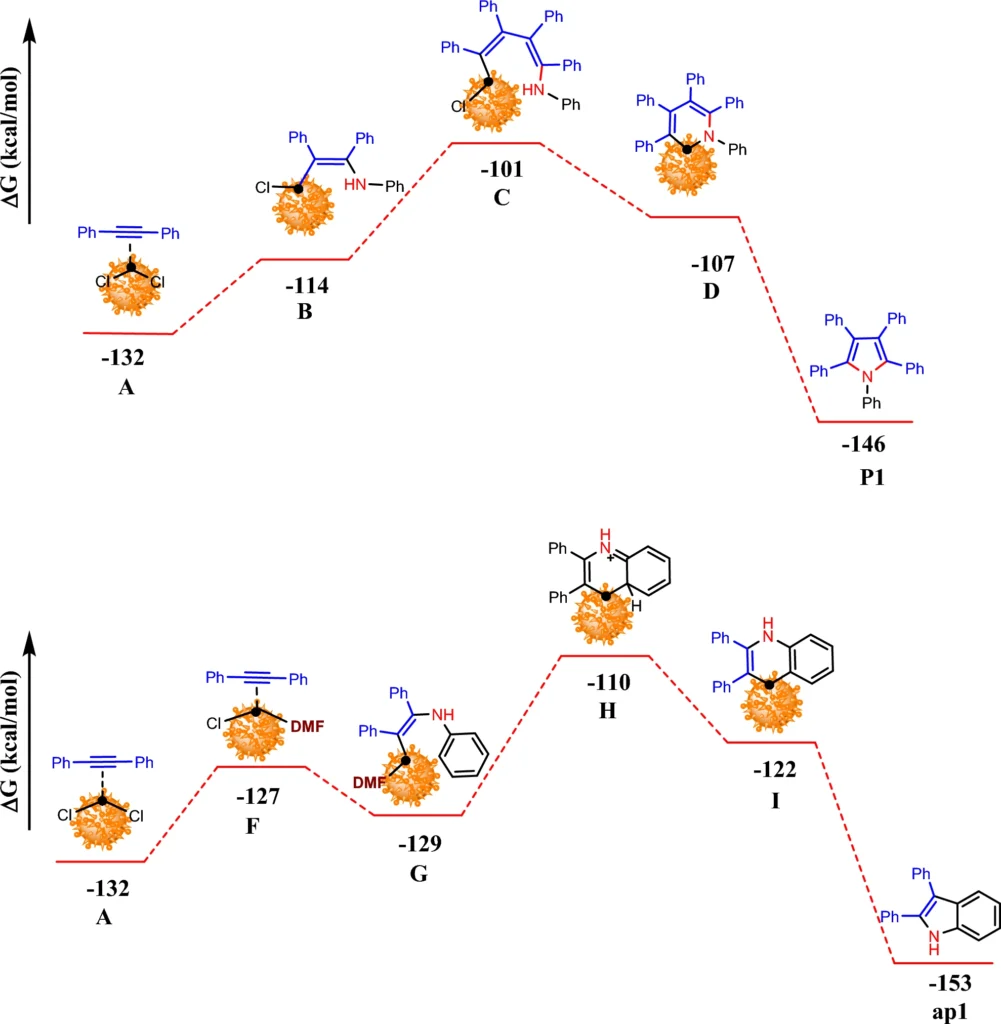
An overall comparison for the changes of Gibbs free energies of ap1 and P1 formation in DMF and 1,4-dioxane, respectively.
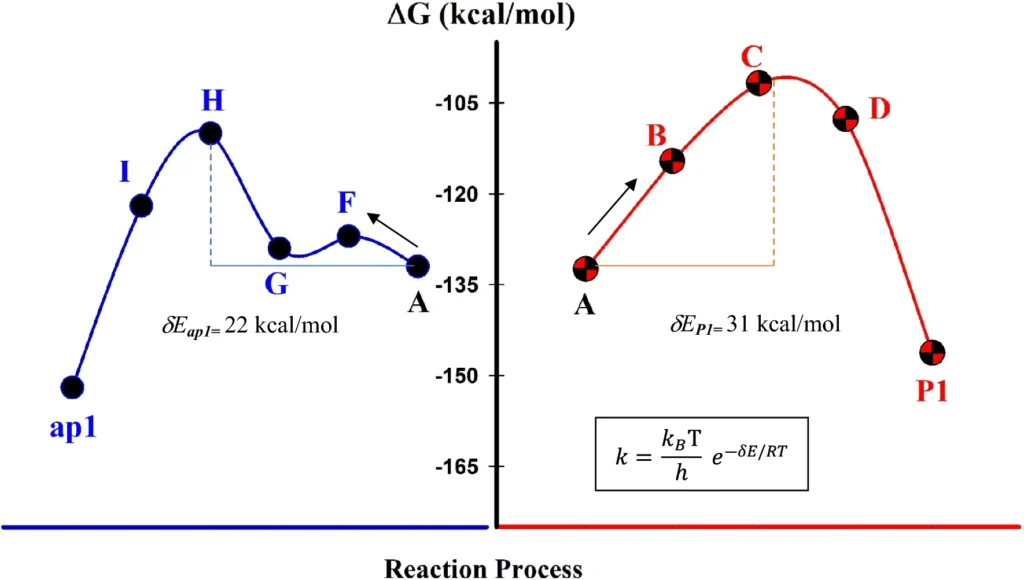
Kinetic investigations and reaction efficacy:
Kinetic experiments have shown that the immobilized palladium catalyst speeds up reaction rates compared to systems that are normally homogeneous. This is due to the elevated surface area and improved contact between the reactants and the catalyst. The solvent additionally influences reaction speeds, as some solvents enhance the process by improving the solvation of reactants.
Comparisons with alternative catalysts:
Instead of other palladium-based catalysts, the triazine dendrimer-supported system is more stable and can be used more than once. Conventional catalysts frequently experience palladium leaching and deactivation over time; however, the dendrimer-based approach addresses these issues well. Furthermore, the magnetic properties facilitate the catalyst’s recovery and reutilization, providing economic benefits.
Obstacles and Constraints:
Despite the numerous benefits, the amplification of palladium-immobilized dendrimer catalysts is associated with certain problems. The dendrimer synthesis and its conjugation to MNPs may be labor-intensive and costly. Moreover, although the catalyst is recyclable, its efficacy may somewhat diminish with prolonged usage. Calculated transition state diagrams for the two reaction pathways.
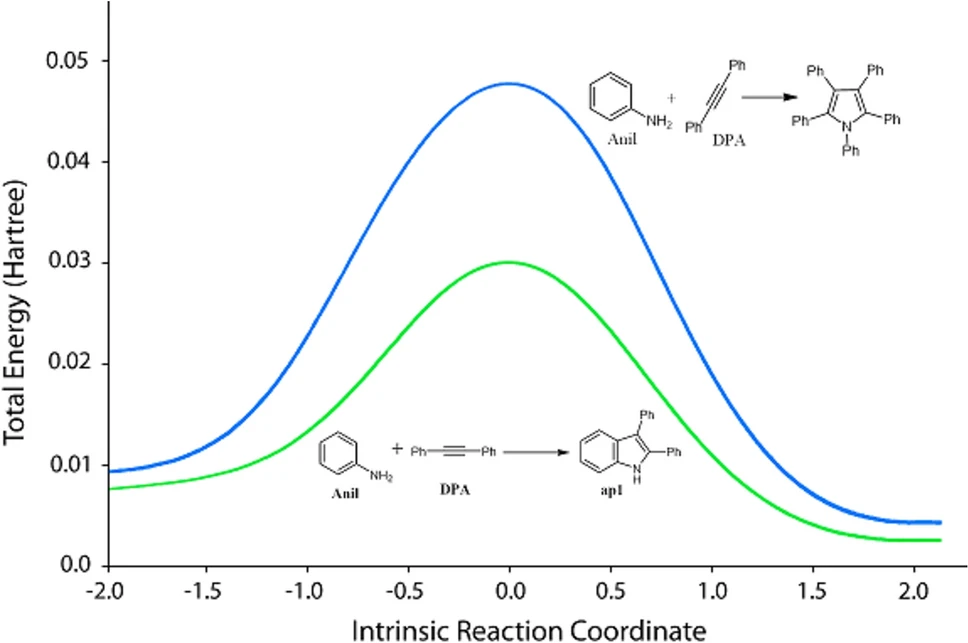
Applications in Organic Synthesis:
Palladium-immobilized triazine dendrimers can be used in a lot of different chemical reactions besides making 2-3-diphenylindoles and pentaphenylpyrrole They have demonstrated potential in cross-coupling reactions, hydrogenation, and intricate multi-step syntheses.
Green Chemistry and Sustainable Catalysis:
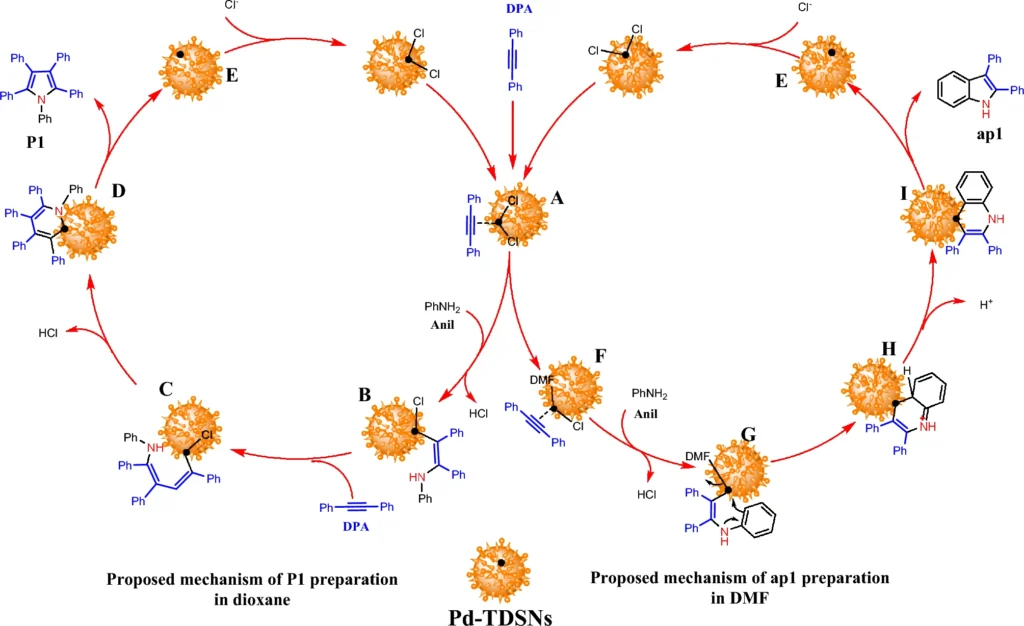
This catalytic system is in complete accordance with the principles of green chemistry. Palladium-immobilized triazine dendrimers on MNPs improve the environmental friendliness of chemical processes by making them reusable, reducing the need for harmful chemicals, and making reaction pathways more efficient. Scheme 7: Proposed mechanism of the formations of P1 and ap1 from diphenylacetylene and aniline catalyzed by Pd-TDSN
Summary:
To sum up, palladium-immobilized triazine dendrimers on magnetic nanoparticles make a useful, reusable microreactor system that is very helpful for methods that depend on solvents for synthesis. Using them to make 2-3-diphenylindoles and pentaphenylpyrrole derivatives shows how useful they are in organic synthesis, and the fact that they can be used again and again is in line with green chemistry principles. As research in this domain advances, these catalysts may become increasingly essential to sustainable and efficient chemical processes.
Frequently Asked Questions:
1). What attributes make triazine dendrimers suitable for palladium immobilization?
Triazine dendrimers have a very branching structure and a lot of surface area, which makes them perfect for immobilizing palladium and improving the reaction between the catalyst and reactants.
2). What is the solvent’s impact on the synthesis of indoles and pyrroles?
The choice of solvent has an impact on reaction speeds, yields, and selectivity. We frequently employ polar solvents to facilitate electron transport and maximize product yield.
3). Is this catalyst applicable to additional reaction types?
Triazine dendrimers immobilized with palladium can be used in many chemical processes, including cross-coupling and hydrogenation.
4). What is the palladium-immobilized catalyst’s reusability?
The catalyst is reusable for several cycles, generally up to 10 or more, with a negligible decline in catalytic efficacy.
5). What are the benefits of employing magnetic nanoparticles in catalysis?
Magnetic nanoparticles allow for straightforward catalyst retrieval via a magnet, reducing the need for filtration and improving reusability.
For more chemistry blogs, visit chemistry Master