Table of Contents
1. Preface
Bisnitroepoxides are an important group of organic compounds that are widely used to make sulfur-containing compounds like bis(thiazolidine-2-thiones), bis(dithiocarbamates), bis(xanthates), and bis(thiazoles). These derivatives possess substantial applicability across multiple domains, including medicines, agriculture, and polymer chemistry. Because nitro and epoxide functional groups are present in bisnitroepoxides, they are naturally reactive. This makes them useful as building blocks for making complex molecular structures. This article investigates the production of bisnitroepoxides, their role in producing sulfur-rich derivatives, and the potential applications of these chemicals.
This talk will examine the production pathways of bisnitroepoxides, the reaction processes for their derivatives, and the significance of these chemicals in diverse industrial areas. We will emphasize the reaction conditions, the difficulties encountered during synthesis, and prospects for research and development in this domain.
2. What are bisnitroepoxides?
Bisnitroepoxides are a unique group of molecules that can be identified by their nitro groups (-NO₂) and epoxide rings, which are made up of three cyclic ethers. These functional groups make bisnitroepoxides highly reactive, positioning them as superior intermediates in organic synthesis. Since there is tension in the epoxide ring, bisnitroepoxides can easily take part in nucleophilic ring-opening events. This allows for the creation of more complex structures.
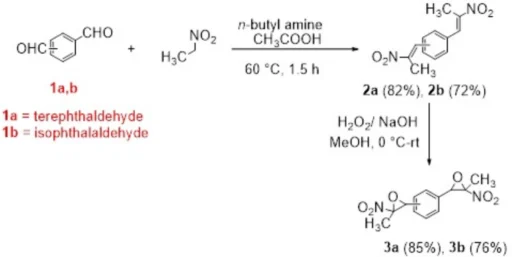
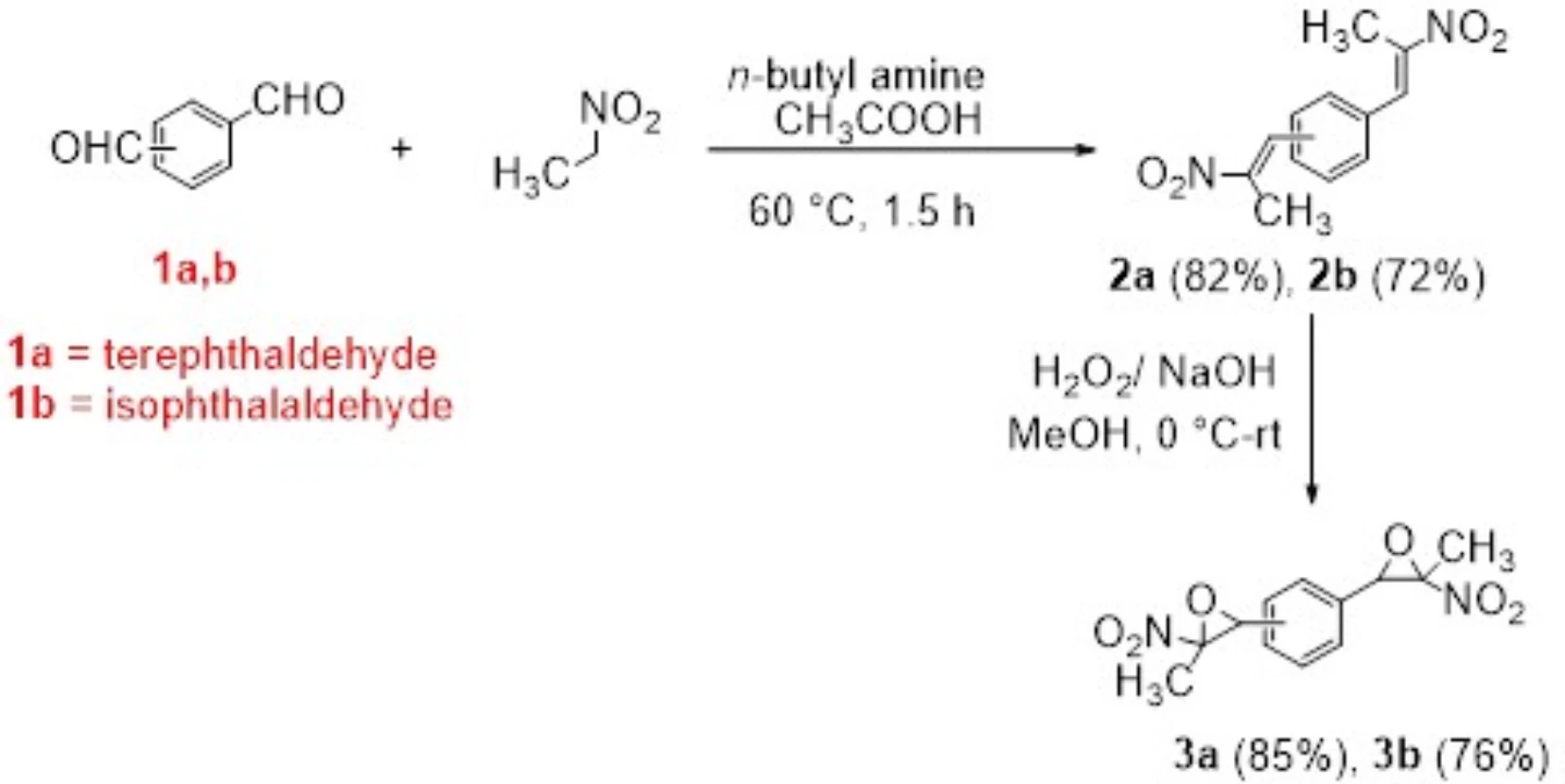
The best thing about bisnitroepoxides is that they can help make sulfur-containing heterocyclic compounds, which are important in many fields, such as materials research and medical chemistry. These compounds can engage in various chemical transformations, especially interactions with nucleophiles such as thiols, amines, and carbanions. This reactivity facilitates the efficient synthesis of derivatives including bis(thiazolidine-2-thiones), bis(dithiocarbamates), bis(xanthates), and bis(thiazoles). Synthesis of bis(nitroepoxides) 3a and 3b from terephthaldehyde (1a) and isophthalaldehyde (1b).
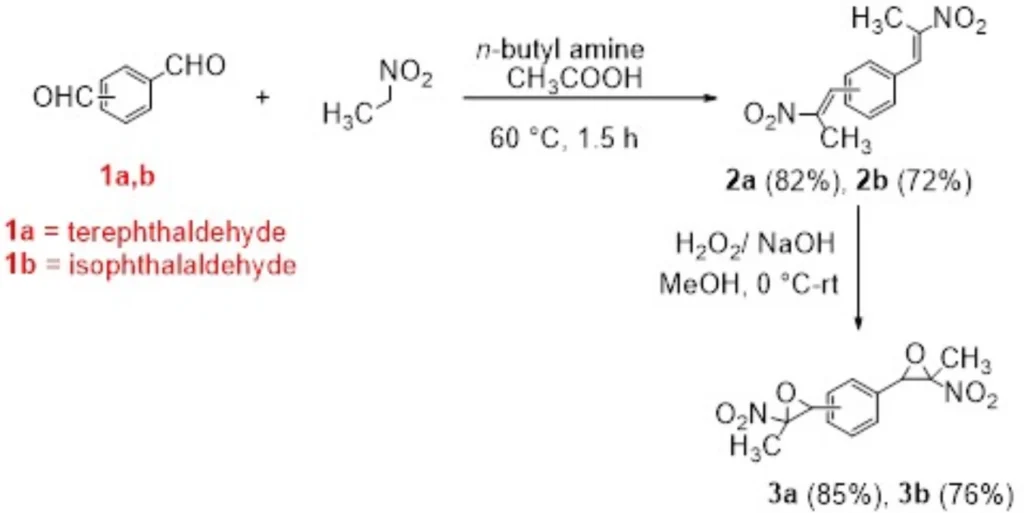
3. Synthesis of Bisnitroepoxides
The production of bisnitroepoxides typically entails the interaction of alkenes with nitrating agents, such as dinitrogen tetroxide (N₂O₄) or a combination of nitric acid (HNO₃) and acetic anhydride. Alkenes undergo nitration to produce nitroalkenes, which undergo epoxidation to transform into bisnitroepoxides. The following is a detailed technique for producing bisnitroepoxides:
Sequential Protocol:
1). Starting materials: The synthesis begins with the use of a suitable alkene, such as 1,3-butadiene, or a comparable diene system.
2). Nitration: A nitrating agent, such as N₂O₄ or a mix of HNO₀ and acetic anhydride, is added to the alkene to add nitro groups to the double bonds. This reaction generally transpires under low temperatures (-5 to 0 °C) to regulate reactivity and prevent breakdown.
3). Epoxidation: An epoxidizing agent, such as m-chloroperoxybenzoic acid (mCPBA), subsequently reacts with the nitroalkene from the nitration process to yield the bisnitroepoxide. The epoxidation reaction transpires in mild circumstances, where temperature and solvent choice are important for enhancing yield and selectivity.
4). Isolation: Once the reaction is over, the bisnitroepoxide product is cleaned up using methods like vacuum distillation, recrystallization, or column chromatography, depending on the compound’s properties (for example, its boiling point and solubility).
4. Mechanism of Bisnitroepoxide Synthesis
Nitration and epoxidation are the two main phases of the bisnitroepoxide synthesis process. Comprehending these mechanisms is crucial for enhancing reaction conditions and increasing yields.
Nitration Mechanism:
Adding a nitronium ion (NO₂⁺) to the carbon-carbon double bond is what nitration of alkenes is all about. This step leads to the formation of a nitroalkene intermediate. In situ production of the nitronium ion occurs from dinitrogen tetroxide (N2O3) or nitric acid in the presence of acetic anhydride.
Mechanism of Epoxidation:
When the nitroalkene is made, the epoxidation reaction takes place through a coordinated process that adds an oxygen atom to the double bond. Epoxidizing chemicals, including mCPBA and hydrogen peroxide (H₂O₂), are frequently employed for this purpose. The reaction’s stereoselectivity is crucial, as it dictates the configuration of the resultant epoxide ring.
5. Utilizations of Bisnitroepoxides
Bisnitroepoxides have a wide range of applications in organic synthesis, particularly in the formation of sulfur-containing derivatives. Their reactivity allows them to participate in numerous nucleophilic substitution processes, resulting in the synthesis of heterocyclic compounds with considerable industrial and medicinal significance.
What is the significance of bisnitroepoxides?
Versatility: Bisnitroepoxides can react with different nucleophiles, like thiols, amines, and alkoxides. This makes them useful building blocks for making different chemical structures.
Sulfur-rich compounds: Bisnitroepoxides can produce a variety of sulfur-rich compounds, such as bis(thiazolidine-2-thiones), bis(dithiocarbamates), and bis(xanthates). These chemicals have significant uses in agriculture, materials science, and pharmacology.
Efficient synthesis: Bisnitroepoxides offer an effective method for creating intricate molecular frameworks with minimal steps, rendering them advantageous for industrial-scale production.
6. Synthesis of Bis(thiazolidine-2-thiones)
Bis(thiazolidine-2-thiones) are sulfur-containing heterocyclic compounds that have garnered attention for their potential applications in medical chemistry. Under moderate circumstances, bisnitroepoxides react with thioamides to produce these chemicals.
Mechanism of Reaction:
1). Nucleophilic attack: As a nucleophile, the sulfur atom in the thioamide goes after the carbon atom in the epoxide ring of the bisnitroepoxide that doesn’t have enough electrons.
2). Ring opening: Nucleophilic assault on the epoxide ring results in its opening and the creation of an intermediate molecule.
3). Cyclization: The intermediate subsequently undergoes cyclization, resulting in the formation of the thiazolidine ring structure.
4). Thione formation: The penultimate step is the production of the thione functional group (C=S), so the synthesis of bis(thiazolidine-2-thiones) is completed.
Typically, we conduct the reaction at ambient temperature in a polar solvent like ethanol or acetonitrile. Regulating the reaction duration, temperature, and reagent stoichiometry can enhance the yields of bis(thiazolidine-2-thiones).
Utilizations of Bis(thiazolidine-2-thiones):
Pharmaceuticals: Bis(thiazolidine-2-thiones) have the potential to be antibacterial and anti-inflammatory agents, making them viable candidates for therapeutic development.
Materials science: The development of innovative materials with distinctive electrical and optical properties may potentially utilize these compounds. Synthesis of bis-thiazole-2(3H)-thiones from bis(nitroepoxides). aIsolated yield. reaction conditions: amine (3.6 equiv), CS2 (6 equiv) in EtOH (0.1 M) for 30 min. at rt, then 3a or 3b
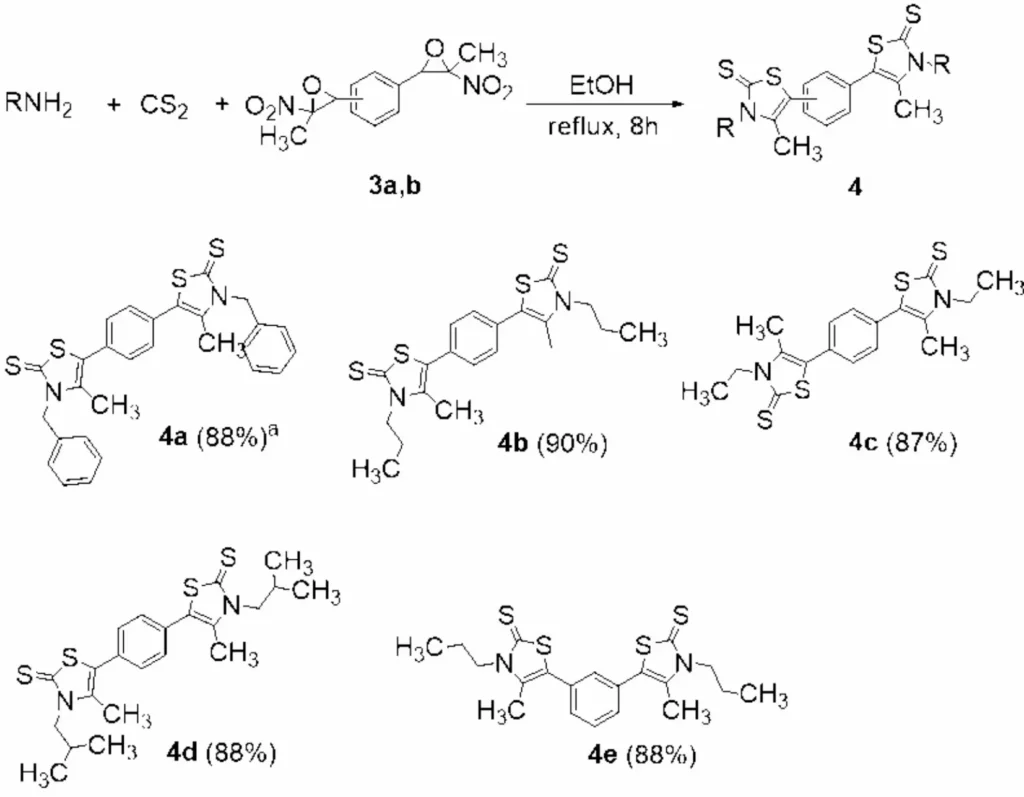
7. Synthesis of Bis(dithiocarbamates)
Dithiocarbamates are sulfur-containing chemicals that are widely used in agriculture as fungicides and in the rubber industry as vulcanization accelerators. A process called bisnitroepoxides interaction with secondary amines and carbon disulfide (CS₂) is needed to make bis(dithiocarbamates).
Mechanism of Reaction:
Nucleophilic attack by the amine: The secondary amine performs a nucleophilic attack on the epoxide ring, opening it and forming an amine-substituted intermediate.
Reaction with CS₂: The intermediate then interacts with carbon disulfide, creating the dithiocarbamate structure.
Generally, we conduct this reaction at ambient temperature in a polar solvent like DMF (dimethylformamide) or DMSO (dimethyl sulfoxide). Under these circumstances, the reaction proceeds swiftly, often yielding crystalline solids as the resultant bis(dithiocarbamates).
Utilizations of Bis(dithiocarbamates):
Agriculture: Bis(dithiocarbamates) serve as potent fungicides, safeguarding crops from fungal infections.
Rubber industry: The rubber industry uses these compounds as accelerators in the vulcanization process, enhancing the softness and durability of rubber products. Diversity in the synthesis of bis(dithiocarbamates) from bis(nitroepoxides). aIsolated yields. bReaction conditions: Secondary amine (3.6 equiv), CS2 (6 equiv) in ethanol (0.1 M) for 30 min at room temperature, then bis(nitroepoxide)
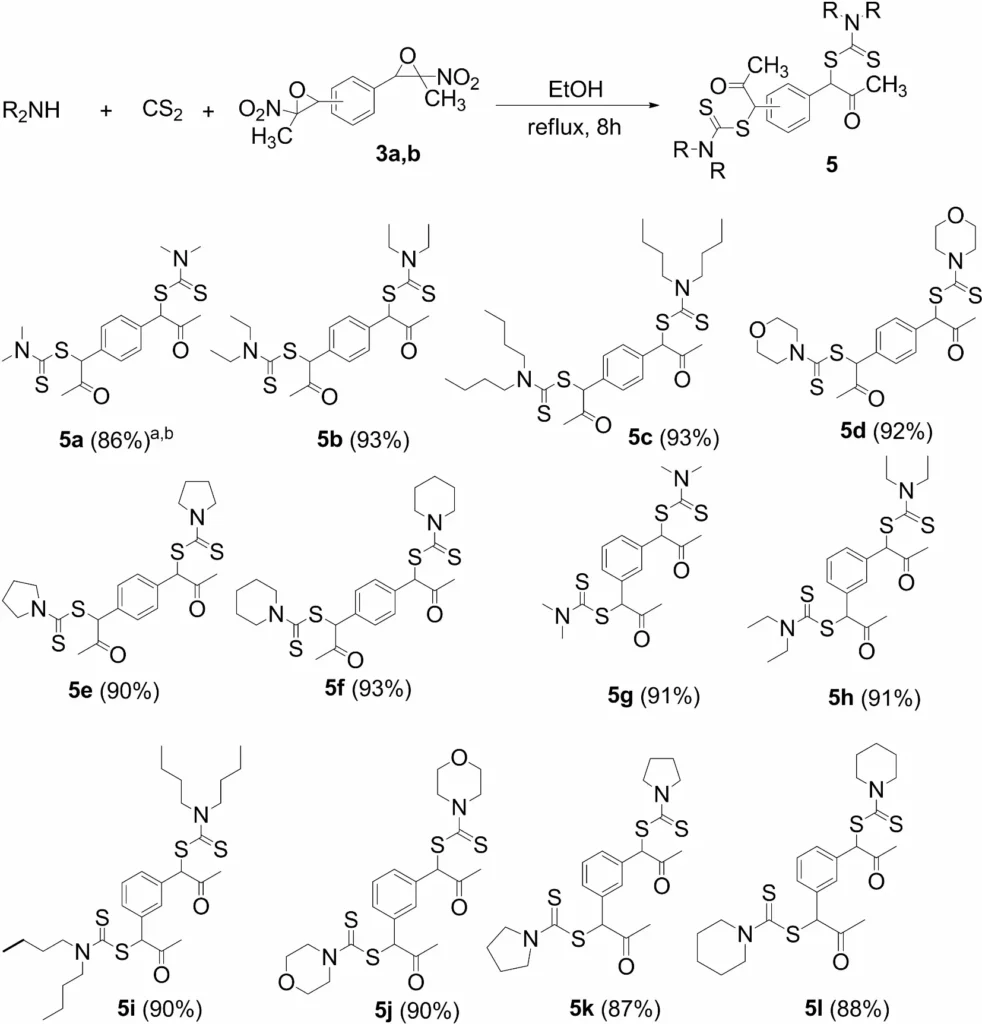
8. Synthesis of Bis(xanthates)
Xanthates are sulfur-containing chemicals that play an important role in polymer chemistry and serve as flotation agents in the mining industry. Reacting bisnitroepoxides with alcohols in the presence of carbon disulfide (CS) can produce bis(xanthates).
Mechanism of Reaction:
Nucleophilic attack by alcohol: When the alcohol attacks the epoxide ring of the bisnitroepoxide, it acts as a nucleophile and creates an alkoxy-substituted intermediate.
Reaction with CS₂: The intermediate subsequently interacts with carbon disulfide to produce the xanthate ester.
This reaction typically occurs in a polar solvent, such as acetone or methanol, with the temperature maintained at a low level to avoid side reactions. You can purify the resultant bis(xanthates) using recrystallization or column chromatography.
Utilizations of Bis(xanthates):
Flotation agents: The mining sector uses bis(xanthates) to aid in the flotation of minerals, particularly sulfide ores.
Polymer chemistry: These chemicals serve as intermediates in the formation of polymers with distinctive characteristics. Diversity in the Synthesis of bis(xanthates). a Isolated yield. b Reaction conditions: potassium xanthate (2.4 equiv), bis(nitroepoxide)
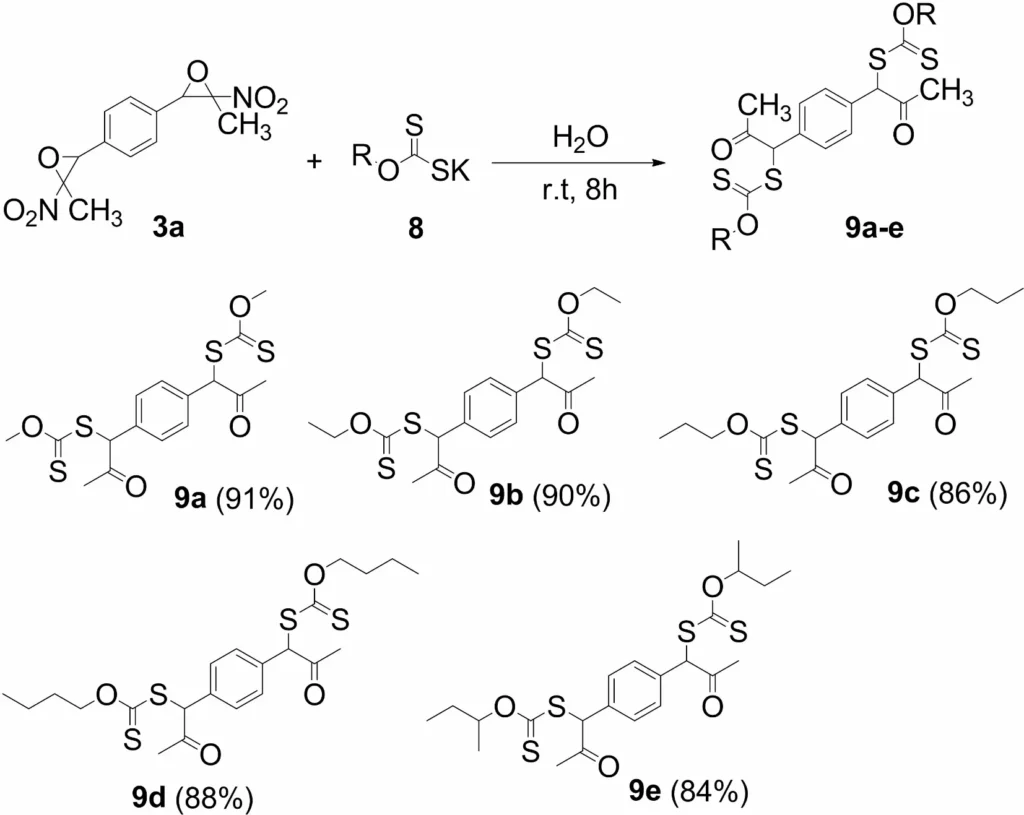
9. Synthesis of Bis(thiazoles)
Thiazoles are heterocyclic molecules containing nitrogen and sulfur, significant in therapeutics and materials research. Bisnitroepoxides react with amines and sulfur-containing compounds to produce bis(thiazoles).
Mechanism of Reaction:
Nucleophilic attack by amine: When the amine attacks the epioxide ring with its nucleophilic force, an amine-substituted intermediate is made.
Cyclization with sulfur: Following this, the intermediate joins with a sulfur-containing reagent (such as elemental sulfur or thiourea), which creates the thiazole ring structure.
Typically, we conduct the reaction in a polar solvent like ethanol or acetonitrile, regulating the temperature to maximize the yield. Typically, we use recrystallization or chromatography to purify the bis(thiazole) compounds.
Utilizations of Bis(thiazoles):
Pharmaceuticals: Pharmaceutical formulations utilize thiazole derivatives for diverse therapeutic applications, including antibacterial and anticancer medicines.
Advanced materials: Bis(thiazoles) are used to create materials with innovative electrical and optical properties, particularly in organic electronics. Synthesis of bis(thiazoles) from bis(nitroepoxides). aIsolated yields. bReaction conditions: S-alkyl dithiocarbamates (2 equiv), bis(nitroepoxide)
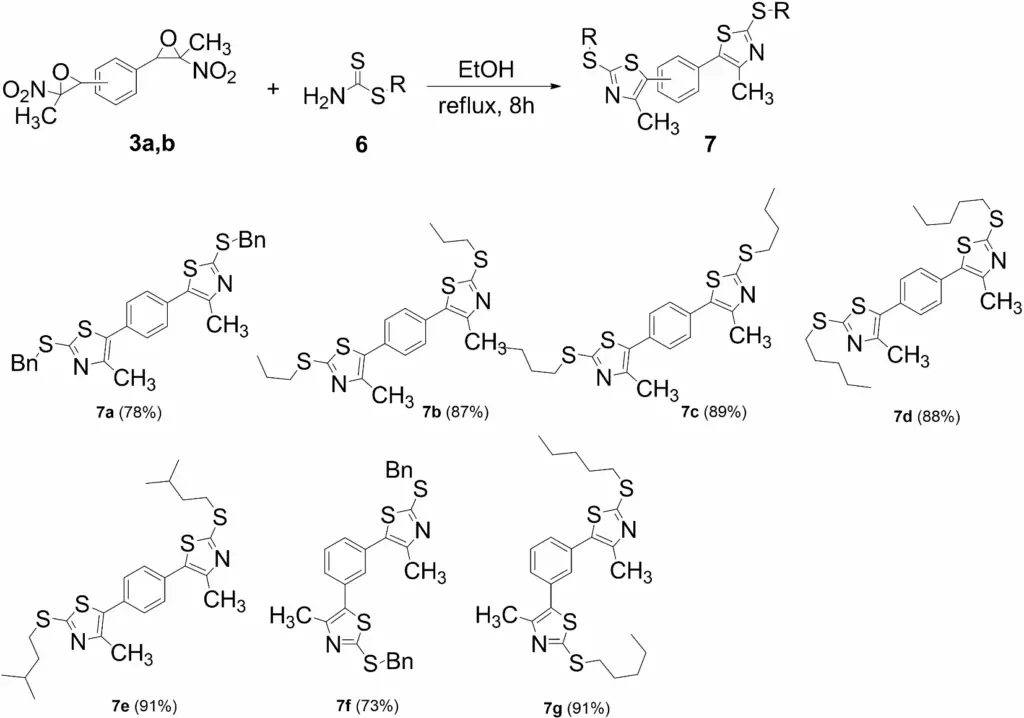
10. Reaction Mechanisms of Each Derivative
Creating bis(thiazolidine-2-thiones), bis(dithiocarbamates), bis(xanthates), and bis(thiazoles) involves attacking the epoxide ring of bisnitroepoxides with a nucleophile. Each derivative follows a different pathway, which is determined by the nucleophile that is used.
Bis(thiazolidine-2-thiones): The nucleophile in bis(thiazolidine-2-thiones) is a thioamide, which makes a thiazolidine ring with a thione functional group.
Bis(dithiocarbamates): A secondary amine acts as the nucleophile in bis(dithiocarbamates), which then reacts with C₂ to form the dithiocarbamate structure.
Bis(xanthates): The nucleophile is an alcohol, which subsequently reacts with C₂ to yield the xanthate ester.
Bis (thiazoles): An amine acts as the nucleophile, then undergoes cyclization with sulfur to yield the thiazole ring.
11. Selection of Solvent and Catalyst
The choice of solvent and catalyst is critical to the efficacy of these reactions. We frequently employ polar solvents such as DMF, DMSO, ethanol, and acetonitrile to promote nucleophilic assaults on the bisnitroepoxide ring. These solvents facilitate the stabilization of reaction intermediates and enhance the solubility of the reagents.
We can use catalysts like Lewis acids (e.g., ZnCl2) or bases (e.g., NaOH) to speed up reactions and increase product yields. The choice of catalyst is dependent on the specific reaction and the intended product.
12. Product Purification and Characterisation
Upon synthesis of the desired derivatives, purification is necessary to eliminate impurities or byproducts. Prevalent purifying techniques encompass:
Recrystallization: We use this technique for substances that are solid at room temperature. The process entails dissolving the compound in a solvent, which then allows it to crystallize when the solvent cools.
Column chromatography: We use this method to separate substances based on their polarity. It is especially effective for purifying product mixes.
Vacuum distillation: This method uses low pressure distillation to purify volatile liquids.
We conduct procedures like these to characterize the purified products.
Characterization of the purified products is carried out using techniques such as:
Nuclear Magnetic Resonance (NMR): By analyzing the chemical shifts of atoms within the molecule, NMR spectroscopy determines the structural composition of compounds.
Infrared (IR) Spectroscopy: By quantifying the absorption of infrared radiation, IR spectroscopy elucidates the functional groups within a substance.
Mass Spectrometry (MS): By ionizing a substance and measuring the mass-to-charge ratio of the resultant ions, mass spectrometry (MS) determines the molecular weight and structure of a substance.
X-ray Crystallography: This method elucidates the intricate three-dimensional structure of a compound by examining the diffraction patterns of X-rays as they pass through a crystalline form of the substance.
13. Industrial and Pharmaceutical Applications
The derivatives of bisnitroepoxides possess extensive uses in multiple fields, notably in agriculture, pharmacology, and materials research.
Utilizations of Bis(thiazolidine-2-thiones):
Antimicrobial agents: Bis(thiazolidine-2-thiones) exhibit antimicrobial properties, positioning them as viable options for disinfectants and antibacterial therapies.
Anti-inflammatory drugs: These chemicals have demonstrated efficacy as anti-inflammatory medicines, with prospective applications in the management of ailments such as arthritis and other inflammatory disorders.
Utilizations of Bis(dithiocarbamates):
Agricultural fungicides: Agriculture extensively uses bis(dithiocarbamates) as fungicides to protect crops from fungal diseases. They are especially efficacious against mold and rust.
Rubber industry: In the rubber business, bis(dithiocarbamates) serve as vulcanization accelerators, enhancing the suppleness and durability of rubber products.
Utilizations of Bis(xanthates):
Flotation agents: The mining sector uses bis(xanthates) as flotation agents to help separate sulfide ores from other minerals during the flotation process.
Polymer synthesis: Xanthates serve as intermediates in the production of polymers with distinctive characteristics, particularly biodegradable plastics.
Utilizations of Bis(thiazoles):
Pharmaceuticals: The pharmaceutical industry extensively uses thiazole derivatives to develop drugs that target a variety of therapeutic domains, including antibacterial, antiviral, and anticancer agents.
Advanced materials: Organic semiconductors and light-emitting diodes (LEDs), in particular, use bis(thiazoles) to create materials with innovative electrical capabilities. Proposed mechanism for the synthesis of products
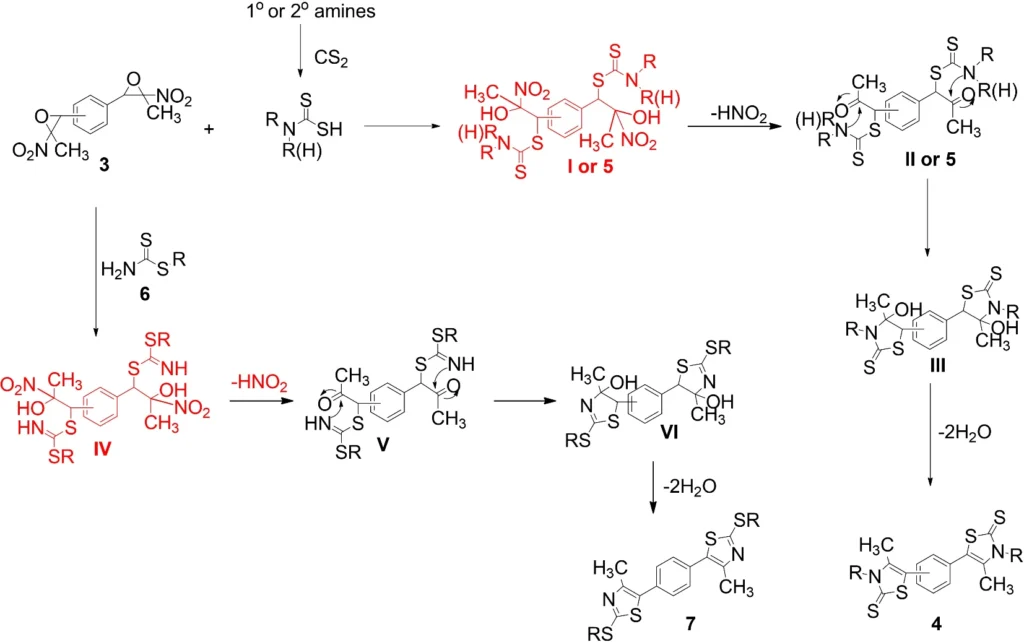
14. Obstacles in Synthesis
Despite the usefulness of bisnitroepoxides and their derivatives, there are numerous obstacles to their manufacture. Commonly encountered issues include:
Suboptimal yields: In some cases, incomplete reactions or the production of unwanted byproducts may reduce the yields of the target products.
Side reactions: Bisnitroepoxides exhibit strong reactivity, potentially resulting in side reactions that generate unwanted chemicals. Regulating the reaction parameters, including temperature, solvent selection, and reagent concentrations, is crucial for reducing unwanted side reactions.
Reactivity of intermediates: The intermediates generated during the reaction may exhibit instability, resulting in challenges in the isolation and purification of the end products. Meticulous tuning of the reaction parameters is essential to resolving this issue.
15. Prospective Outlooks
Bisnitroepoxide chemistry’s domain continues to develop, with current investigations examining novel uses and derivatives. Possible avenues for future research include:
New sulfur-containing derivatives: Researchers are consistently looking for novel sulfur-containing bisnitroepoxide derivatives for potential use in drug development and materials research.
Green chemistry methodologies: There is an increasing focus on creating more eco-friendly synthesis techniques for bisnitroepoxides and their derivatives. This encompasses investigating renewable reagents, minimizing harmful solvent usage, and enhancing the overall efficiency of reactions.
Biomedical applications: Bisnitroepoxide derivatives are under investigation for their potential as bioactive agents, especially in the formulation of novel therapeutics for infectious illnesses and cancer. These molecules’ distinctive chemical characteristics make them interesting candidates for future medicinal uses.
Advanced materials: Researchers are looking into bisnitroepoxide derivatives for potential uses in the creation of novel materials, such as organic semiconductors and solar devices.
16. Summary
To sum up, bisnitroepoxides are very reactive building blocks that are needed to make sulfur-containing compounds like bis(thiazolidine-2-thiones), bis(dithiocarbamates), bis(xanthates), and bis(thiazoles). These derivatives have extensive uses in agriculture, medicine, and materials research. The synthesis of these compounds entails nucleophilic attacks on the epoxide ring, followed by a variety of cyclization or addition processes depending on the nucleophile used. Regardless of the difficulties associated with their production, persistent research is investigating novel applications and derivatives of bisnitroepoxides, presenting promising prospects for future advancements in both industry and academia.
17. Frequently Asked Questions
1). What are the applications of bisnitroepoxides?
Bisnitroepoxides serve as organic synthesis intermediates for the production of sulfur-containing chemicals used in agriculture, medicines, and materials science.
2). What methods can enhance the yields of bis(thiazolidine-2-thiones)?
Adjusting reaction parameters such as temperature, solvent selection, and reagent concentrations can enhance yields. Using a catalyst can improve reaction efficiency.
3). What are the industrial applications of bis(dithiocarbamates)?
Bis(dithiocarbamates) serve as fungicides in agriculture to safeguard crops from fungal infestations. The rubber sector uses them as accelerators for vulcanization.
4). Are bis(xanthates) hazardous?
If improperly managed, bis(xanthates) may exhibit toxicity, particularly in substantial amounts. We must adhere to proper safety protocols when handling these substances.
5). What potential future applications exist for bis (thiazoles)?
Bis(thiazoles) may possess prospective applications in the formulation of novel medications and sophisticated materials for organic electronics and semiconductors.
For more chemistry blogs, visit chemistry Master