Table of Contents
Preface:
Methamphetamine, often referred to as meth, is a potent stimulant of the central nervous system, recognized for its significant addictive potential. Methamphetamine abuse constitutes a substantial public health concern worldwide because of its detrimental effects on the brain and body. Understanding the evolution of drug dependence is essential for properly combating addiction. Metabolomics, the study of tiny chemicals known as metabolites, is a powerful approach to examining these alterations. By integrating targeted and non-targeted metabolomics methodologies, researchers can obtain an enhanced understanding of the biochemical changes linked to drug dependence.
Rat Model for Methamphetamine Self-Administration:
The methamphetamine self-administration rat model is a prevalent instrument in addiction research. This approach enables rats to freely Methamphetamine Self-Administration, replicating human drug consumption behavior. It offers a regulated method to examine the progression of drug consumption behaviors across time. Through the observation of rats in this setting, researchers may investigate the phases of addiction and discern behavioral alterations that reflect human drug dependency.
The Methamphetamine Self-Administration model is particularly significant as it accurately mirrors the human experience of addiction, encompassing the shift from recreational usage to compulsive drug-seeking behavior. Rats subjected to methamphetamine demonstrate heightened motivation to ingest the substance, resembling the cravings and drug-seeking behaviors observed in people.
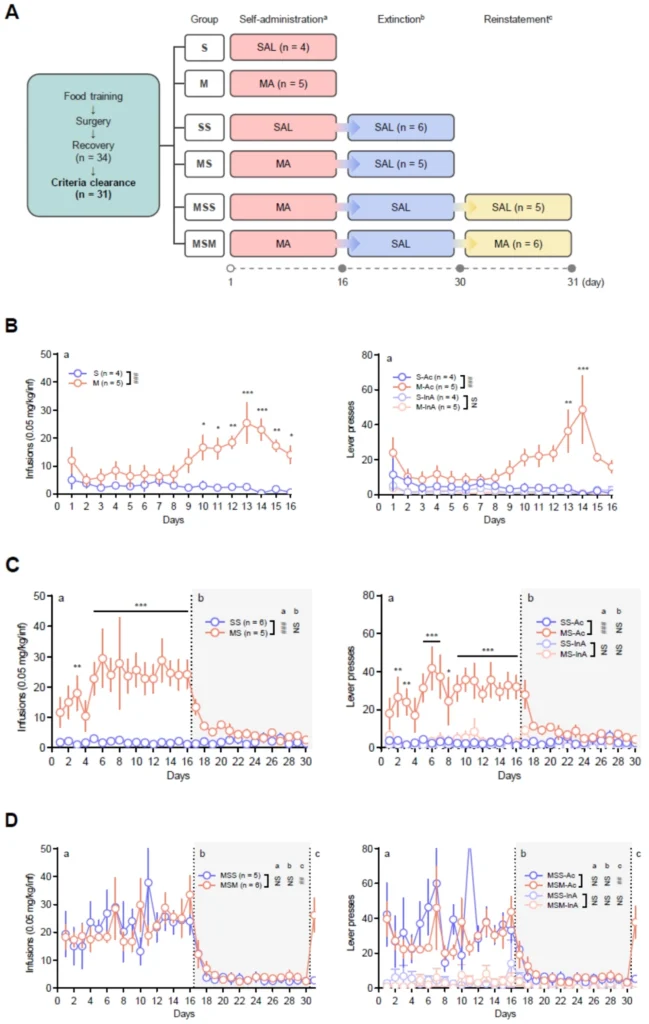
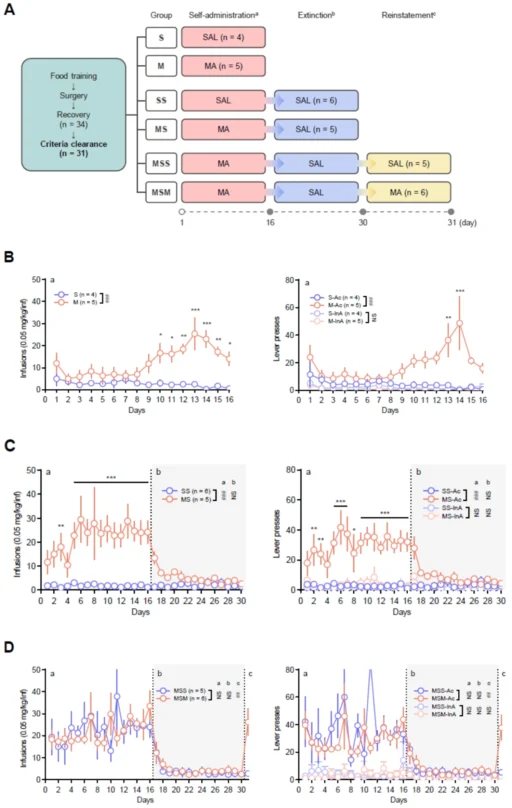
(A) Timeline, experimental sessions, and animal groups for behavioral assessment. A gray circle represents the day of blood collection. (B) Numbers of infusions and lever presses during the self-administration period (1–16 days) of Groups S and M. (C) Numbers of infusions and lever presses during self-administration (1–16 days) and extinction period (17–30 days) of Groups SS and MS. (D) Numbers of infusions and lever presses during self-administration (1–16 days), extinction (17–30 days), and reinstatement period (31 day) of Groups MSS and MSM.
Advancement of Substance Dependence:
To distinguish drug misuse from dependency, it is essential to comprehend the subtle alterations that transpire after prolonged methamphetamine exposure. Drug abuse refers to the harmful use of a substance, whereas dependency is defined as a physical or psychological craving for the drug. In the methamphetamine rat model, dependency advances through specific phases. Initially, rats may ingest methamphetamine for recreational purposes, but as dependence develops, their consumption becomes obsessive and relentless, despite adverse repercussions.
Behavioral modifications include heightened drug-seeking behaviors and a decreased interest in intrinsic rewards such as sustenance or social engagement. These alterations underscore the brain’s reconfiguration during methamphetamine dependence.
Metabolomics overview:
Metabolomics is the comprehensive study of tiny chemicals, or metabolites, produced by metabolic processes. In addiction research, metabolomics offers an overview of the biochemical alterations resulting from drug consumption. By monitoring these metabolites, researchers can discern how the body’s biochemistry adjusts to extended drug use, uncovering biochemical indicators of addiction.
Metabolomics encompasses two primary methodologies: targeted and non-targeted. Targeted metabolomics emphasizes the quantification of known compounds, whereas non-targeted metabolomics adopts a broader approach, seeking novel or unforeseen metabolic alterations.
Targeted Metabolomics Methodology:
Targeted Metabolomics, researchers concentrate on specific metabolites linked to distinct biological pathways. This approach is optimal for examining established mechanisms of methamphetamine dependence. Methamphetamine influences dopamine, a principal neurotransmitter, which Targeted Metabolomics can monitor to elucidate its function in reward and addiction.
Targeted metabolomics, by concentrating on particular metabolic pathways, enables researchers to identify biomarkers that may indicate methamphetamine addiction. These biomarkers may ultimately serve to track the progression of dependence or the efficacy of therapeutic therapies.
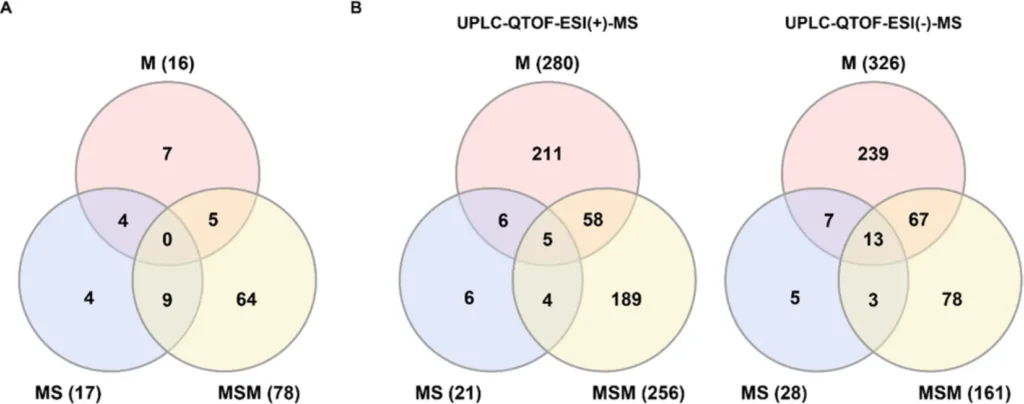
Venn diagrams of the numbers of significantly altered metabolites or ion features. (A) Targeted analysis. (B) Non-targeted analysis
Non-Targeted Metabolomics Methodology:
Conversely, non-targeted metabolomics entails the thorough examination of all identifiable metabolites inside a biological specimen. This methodology is especially beneficial for examining a multifaceted condition such as addiction, which impacts various systems. Because of the extensive effects of methamphetamine on the body, non-targeted metabolism provides an impartial perspective on the metabolic changes induced by the substance.
Non-targeted investigations can reveal previously unidentified metabolic pathways associated with addiction, providing a comprehensive understanding of methamphetamine’s impact on the brain and other organ systems.
Metabolomic Alterations During Methamphetamine Consumption:
In the initial phases of methamphetamine consumption, significant alterations transpire in the body’s metabolism. Researchers have noted alterations in neurotransmitter concentrations, namely within dopamine and serotonin pathways. These alterations influence reward processing in the brain, hence encouraging drug-seeking behavior.
Moreover, methamphetamine affects the body’s energy metabolism, elevating oxidative stress and modifying lipid metabolism. Initial metabolomic investigations in rats have demonstrated that these alterations correspond with the intensity of drug-seeking behavior and the severity of dependency.
Persistent Methamphetamine Consumption and Metabolic Alterations:
Prolonged methamphetamine usage induces significant metabolic alterations in the body. Prolonged exposure results in disturbances in energy metabolism, significantly affecting glucose and fatty acid metabolism. These disturbances lead to the physical deterioration frequently observed in chronic methamphetamine users, encompassing weight reduction, cardiovascular complications, and cognitive deficits.
In the rat model, these enduring alterations are discernible using metabolomic analysis. Investigators have discovered that prolonged methamphetamine consumption disrupts neurotransmitter equilibrium, hepatic function, and immunological response, signifying the drug’s systemic effects.
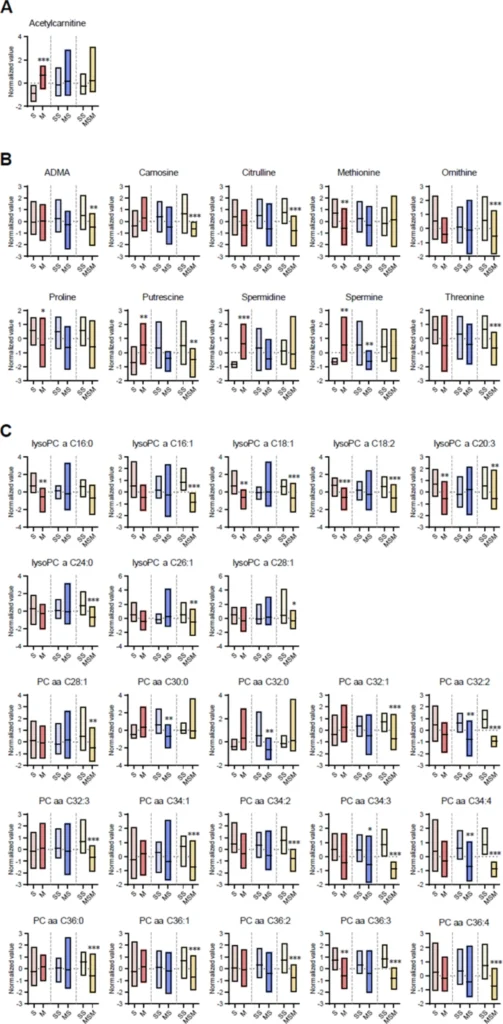
Box plots of the target metabolites in plasma collected from methamphetamine self-administering rats at different stages of drug dependence progression. (A) Acylcarnitine. (B) Amino acids and biogenic amines. (C) Glycerophospholipids. (D) Sphingolipids. (E) TCA intermediates
Correlation between Metabolomics and Behavioral Observations:
One of the most significant features of metabolomics is its capacity to associate biochemical alterations with observable behaviors. Several metabolic indicators strongly correlate with drug-seeking behaviors in rats addicted to methamphetamine. For instance, elevated concentrations of specific neurotransmitters correlate with increased motivation to acquire methamphetamine, while changes in energy metabolism lead to fatigue and cognitive decline.
Biomarkers of Methamphetamine Addiction:
Identifying dependable biomarkers is a primary objective in addiction research. Biomarkers facilitate the early detection of dependence and provide prospective targets for therapeutic intervention. Biomarkers linked to changes in neurotransmitters, oxidative stress, and energy metabolism have come to light as possible future drug targets or diagnostic tools for methamphetamine addiction.
Methamphetamine influences metabolic pathways:
Methamphetamine markedly interferes with multiple metabolic processes. The brain’s reward system relies on the dopamine pathway. Moreover, methamphetamine influences glutamate signaling, which is crucial for cognitive tasks including learning and memory. Other impacted pathways include those related to lipid metabolism, which affects cell membrane integrity and signaling, as well as oxidative stress pathways, which may result in cellular damage over time.
Obstacles of Metabolomics in Addiction Studies:
Despite the significant potential of metabolomics, it faces various obstacles. Addiction is a multifaceted disorder, shaped by genetic, environmental, and behavioral influences. Interpreting metabolic data can be challenging without accounting for these further layers of complexity. Furthermore, technological constraints, such as the sensitivity of detection techniques and the substantial volume of data produced, present hurdles for researchers.
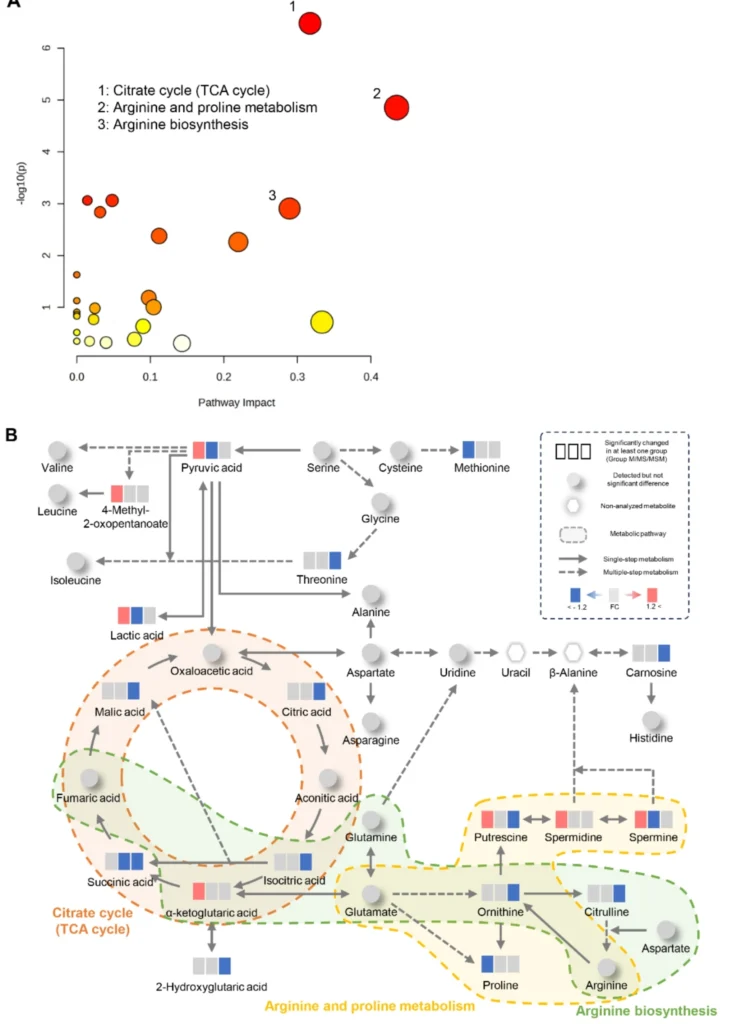
Results of metabolic pathway analysis. (A) Metabolic pathway analysis using MetaboAnalyst. The size and color of each circle represent pathway impact value and p-value, respectively. (B) Alterations in metabolic pathways depend on methamphetamine (MA) dependence progression. Down- and upregulated metabolites are highlighted in blue and red, respectively. The gray color represents no significant change. The colored portions inside the dotted line are significant metabolic pathways. The solid and dotted line arrows represent single-step and multiple-step metabolic pathways between metabolites. TCA, tricarboxylic acid.
The advantages of combining targeted and non-targeted strategies:
Employing both targeted and non-targeted metabolomics enables researchers to capitalize on the advantages of each methodology. Targeted analyses yield exact, quantitative information on certain metabolites, whereas non-targeted analyses present a wider, more exploratory perspective. Collectively, these methodologies offer an extensive overview of the molecular alterations linked to methamphetamine addiction.
Prospective Pathways in Addiction and Metabolomics:
Metabolomics has the potential to transform our comprehension and treatment of addiction. By discovering metabolic patterns linked to drug dependence, researchers can formulate individualized treatment strategies that focus on specific biochemical pathways. Moreover, metabolomics may contribute to forecasting relapse or therapy efficacy, providing a significant resource for clinicians.
Final Assessment:
The development of methamphetamine dependence is intricate; nonetheless, metabolomics provides insight into the molecular alterations that underpin addiction. By integrating targeted and non-targeted methodologies, researchers can achieve a thorough comprehension of the metabolic disturbances induced by methamphetamine. These results offer significant potential for the development of novel biomarkers, therapies, and preventive measures for methamphetamine addiction.
Frequently Asked Questions:
1). What distinguishes targeted metabolomics from non-targeted metabolomics?
Targeted metabolomics concentrates on particular metabolites, whereas non-targeted metabolomics examines a broad spectrum of metabolites without bias.
2). In what ways does metabolomics contribute to understanding addiction?
Metabolomics elucidates the biochemical alterations that transpire in the body and brain during drug consumption, assisting researchers in pinpointing the pathways implicated in addiction.
3). What is the rationale for using rats in the study of methamphetamine addiction?
Rats serve as a regulated model that simulates human drug consumption behavior, enabling researchers to examine the progression of addiction in a systematic setting.
4). Can we use metabolomics to treat methamphetamine addiction?
Although metabolomics is not a treatment per se, it can elucidate biomarkers and metabolic pathways that may facilitate the development of more focused medicines for addiction.
5). Which biomarkers are associated with methamphetamine dependence?
Potential biomarkers of methamphetamine dependence include those linked to dopamine signaling, oxidative stress, and energy metabolism.
For more chemistry blogs, visit chemistry Master