Table of Contents
The interplay between light and matter has captivated scientists for ages. This interaction is essential for various natural and technological activities, such as photosynthesis, solar energy conversion, and innovative material design. In this discipline, a particularly captivating area of research is the investigation of ultra-fast photochemistry in the presence of intense light-matter interactions. Often overlooked but crucial, thermal disorder can significantly impact these interactions, impeding the inhibition of extremely rapid photochemical reactions. This article aims to thoroughly examine this phenomenon’s intricacies, specifically focusing on the significant impact of thermal disorder and its influence on the regime of strong light-matter coupling.
Understanding the mechanisms behind rapid photochemical reactions:
Chemical reactions that occur quickly within a very short period are known as ultra-fast photochemistry. These reactions usually take place in time intervals that range from femtoseconds (10<0x7E>15 seconds) to picoseconds (10<0x7E>12 seconds). These reactions are crucial for numerous vital functions, including the first stages of photosynthesis, vision, and the operation of different photonic devices.
Foundations of Rapid Photochemical Reactions:
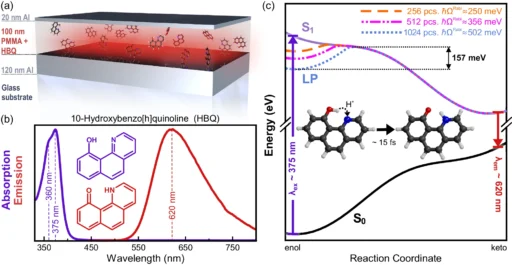
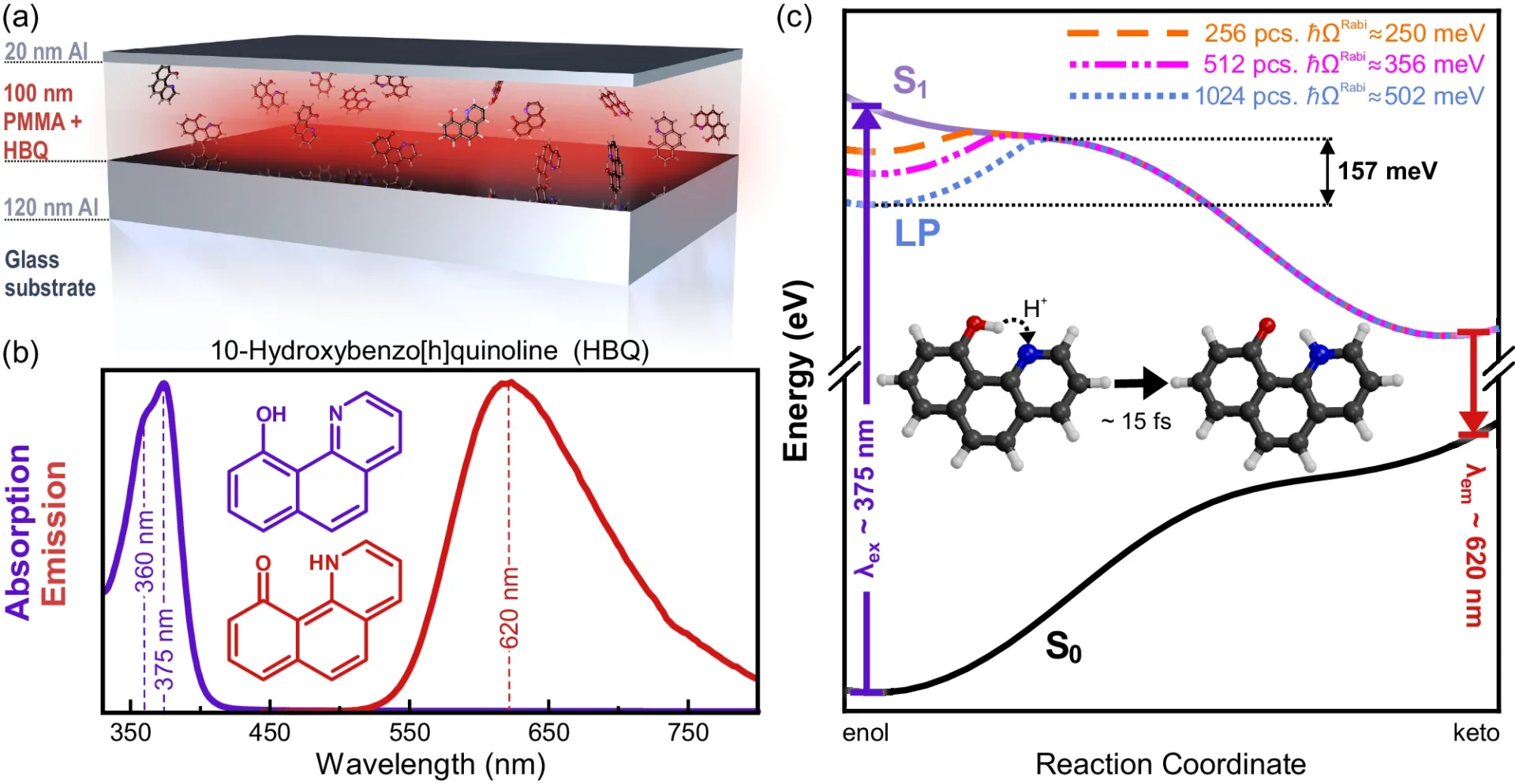
The fundamental aspect of ultra-fast photochemistry revolves around the interplay between light and molecules. When a molecule absorbs a photon, it can transition to a higher electronic state, resulting in excitation. Several elements, including the characteristics of the molecule, its surroundings, and the timeframes involved, determine the outcome of this state of excitement, whether it results in a chemical reaction, fluorescence, or returns to its initial state. Proposed mechanism for suppression of ultra-fast photochemistry due to collective strong coupling in optical cavities.
Applications in diverse fields:
Ultra-fast photochemistry is useful in various fields.
Photosynthesis: A thorough understanding of the rapid processes involved in photosynthesis can lead to the development of more efficient artificial systems for harnessing solar energy.
Vision: The early stages of vision encompass rapid photochemical processes in the retina, which transform light into electrical messages.
Solar cells rely on rapid photochemical processes to effectively absorb light and separate charges in photovoltaic devices.
Medicine: Photodynamic therapy, a cancer treatment, utilizes the principles of photochemistry to eradicate cancer cells.
Regulating photochemical reactions can be difficult:
Controlling ultra-fast photochemical reactions is a tough task, despite its significance. Precision control is challenging due to the extremely short durations and the numerous potential reaction pathways. Scientists have been investigating other approaches to improve control, such as employing intense light-matter interaction.
An overview of the coupling between light and matter:
Light-matter coupling refers to the interaction between electromagnetic waves, such as light, and matter, including molecules and atoms. The strength of this interaction varies depending on the coupling intensity and characteristics.
Principles of Light-Matter Interaction:
Materials can absorb, scatter, or expel light when they come into contact with them. Under weak coupling conditions, these interactions are relatively simple and do not greatly modify the characteristics of the light or the matter. Nevertheless, under the high coupling regime, the interaction is sufficiently powerful to give rise to the creation of novel hybrid states referred to as polaritons.
The characteristics and significance of the strong coupling regime are highlighted:
Strong coupling occurs when light and matter transfer energy so quickly that they can’t be considered separate entities. Instead, they generate novel quasi-particles known as polaritons. They can utilize the distinct characteristics of the polaritons to manipulate photochemical reactions. For example, they can alter the energy profile of a reaction, which might inhibit unwanted routes and promote beneficial ones.
The Significance of Thermal Disorder:
The thermal disorder is the result of thermal energy’s unpredictable movement of molecules and atoms. As the temperature rises, this disorder becomes more evident, resulting in substantial variations in the locations and orientations of molecules.
The Impact of Thermal Disorder on Molecular Systems:
Thermal disorder in molecular systems can cause fluctuations in molecules’ energy levels and states. This disorder can interfere with the optimal conditions required for specific reactions, thereby impeding the achievement of consistent outcomes. Thermal disorder can have a significant impact on the control and predictability of reactions in ultrafast photochemistry.
Illustrations derived from empirical investigations:
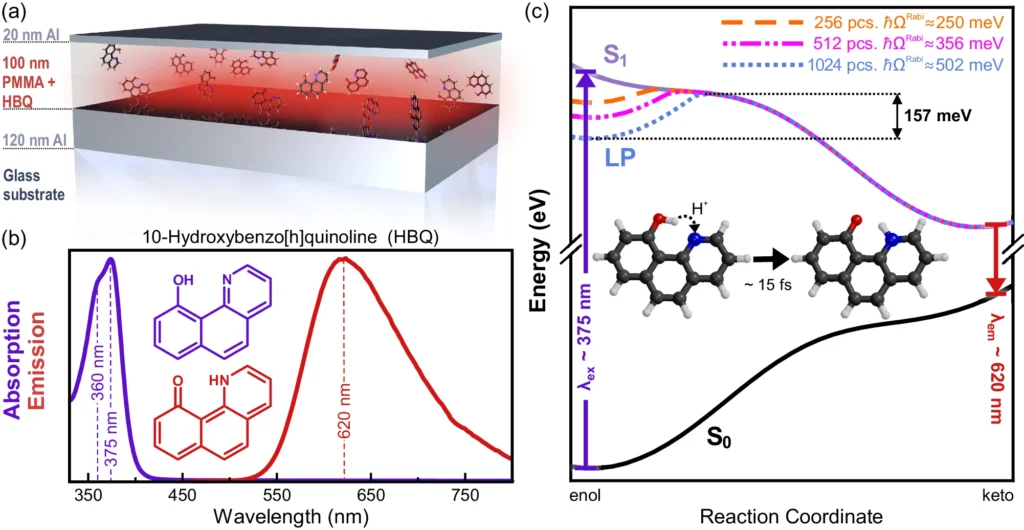
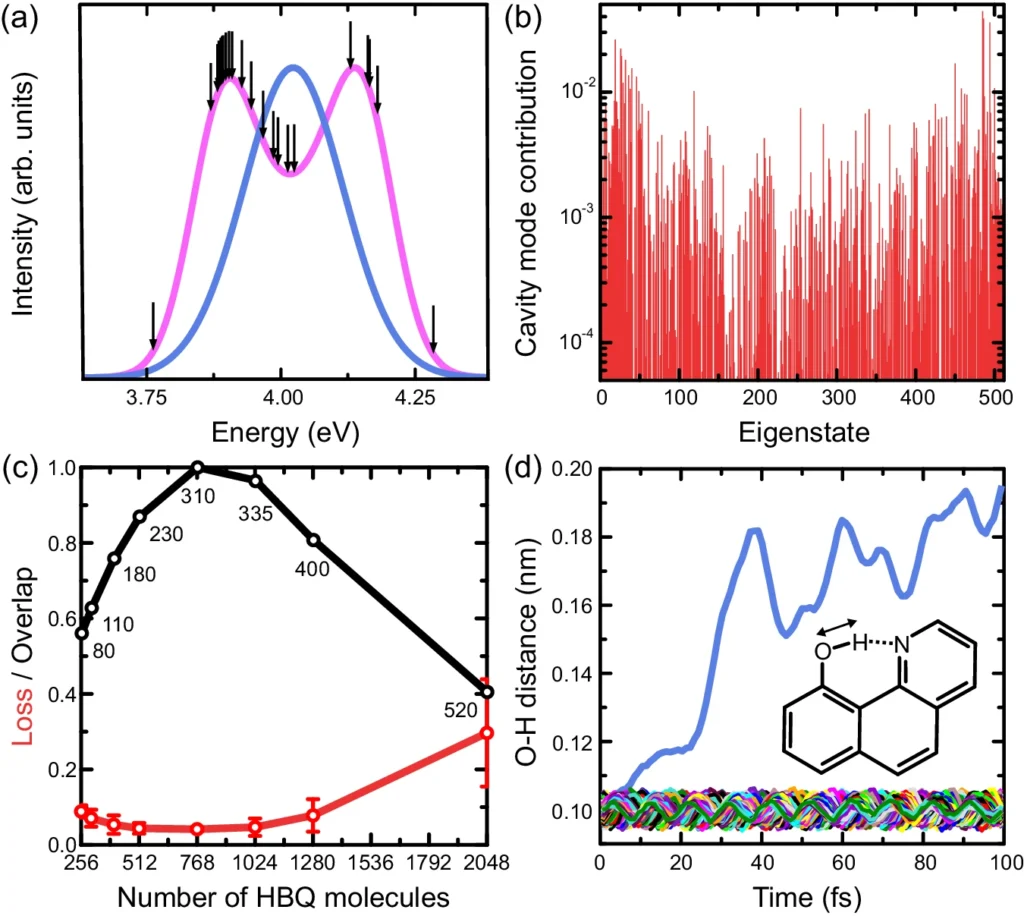
Empirical investigations have demonstrated that thermal disorder can result in the expansion of spectral lines, alterations in reaction speeds, and modifications in reaction pathways. In organic photovoltaic devices, enhanced thermal disorder can diminish the efficacy of charge separation, resulting in decreased device performance. We observe similar phenomena in other systems where meticulous regulation of molecular states is crucial. Results of molecular dynamics simulations of HBQ molecules collectively coupled to a cavity.
The influence of thermal disorder on rapid photochemical reactions:
The thermal disorder causes fluctuations in the energy distribution of molecular systems, which impacts the speed and routes of extremely rapid photochemical processes. The variety of these reactions poses a challenge in terms of prediction and control.
Conceptual Framework:
Theoretical analysis conceptualizes thermal disorder as random variations in the potential energy surface of a molecular system. These variations can modify the comparative energy of reactants, intermediates, and products, resulting in modifications to reaction rates and routes. Even minor fluctuations can have significant consequences in ultra-fast kinetics, where reactions take place within femtosecond time intervals.
Empirical Proof:
Empirical investigations have substantiated the influence of temperature disorder on rapid photochemical reactions. Time-resolved spectroscopic investigations have demonstrated that changing temperatures can induce alterations in reaction dynamics, resulting in either accelerated or decelerated reaction rates, depending on the specific system. The heightened thermal mobility of molecules causes these alterations, modifying the energy configuration and paths of reactions.
Examples illustrate the impact of a specific situation or event:
Multiple case studies demonstrate the influence of temperature disorder on rapid photochemical reactions:
Photosynthetic complexes are susceptible to temperature instability, which can cause fluctuations in the efficiency of energy transfer mechanisms. This, in turn, impacts the overall effectiveness of photosynthesis.
Because of heat instability, organic photovoltaics are susceptible to a decrease in charge separation and transport efficiency, resulting in a decline in device performance.
Photocatalysis involves the use of thermal disorder to modify the speeds of light-induced reactions, which in turn impacts the effectiveness and specificity of catalytic processes.
Regime of Strong Light-Matter Coupling:
Strong light-matter coupling offers intriguing opportunities for manipulating photochemical reactions. However, achieving and maintaining this regimen requires specific conditions that thermal disorder can disrupt.
Definition and Exploration:
In the region of strong coupling, the interaction between light and matter is intense enough to result in the formation of polaritons. These quasi-particles exhibit unique characteristics that enable the manipulation of photochemical reactions. Polaritons can modify the energy distribution of a process, which might inhibit unwanted pathways and enhance beneficial ones.
The benefits of strong coupling:
The strong coupling regime provides numerous benefits for regulating photochemical reactions:
Polaritons can transfer energy within a system, allowing for the possibility of directing it into specific reaction paths.
Strong coupling can augment response rates by amplifying the number of possible states for reaction. Strong coupling can suppress undesirable reactions by altering the energy landscape, which increases selectivity.
Obstacles and Restrictions:
Although it has its benefits, the tight coupling regime also poses difficulties.
To achieve strong coupling, it is necessary to meet certain parameters, such as employing specified cavity designs and using high-quality materials.
The thermal disorder can interfere with the precise circumstances required for strong coupling, thereby impeding the achievement of consistent outcomes.
Polaritons and their interactions with the surrounding environment are characterized by a high level of complexity, which poses difficulties in accurately predicting and controlling reaction outcomes. Measured and modeled dispersions of strongly coupled HBQ cavities.
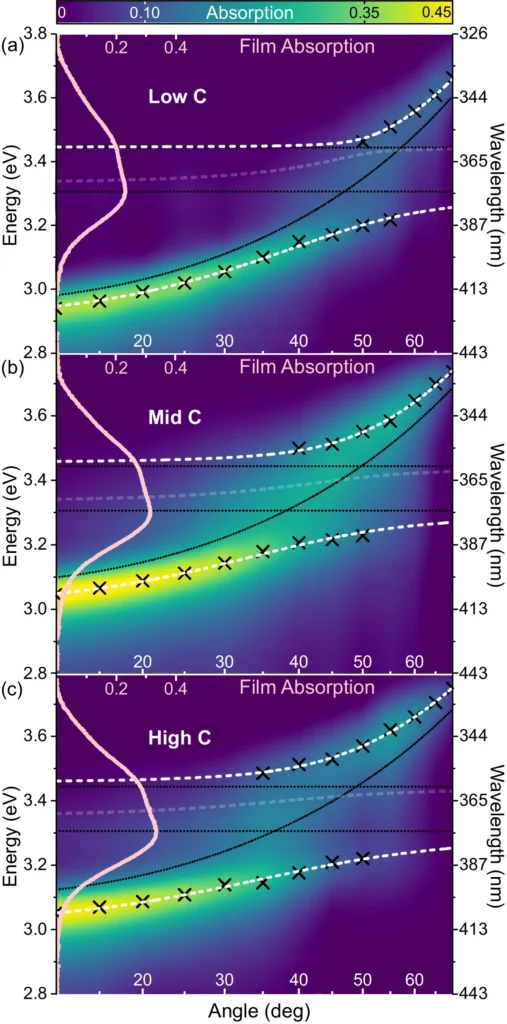
The strong coupling regime inhibits photochemistry:
In theory, the strong coupling regime provides the opportunity to suppress unwanted photochemical reactions by manipulating the energy levels and pathways of the system. Nevertheless, thermal disorder introduces stochastic fluctuations that disturb this regulation.
Anticipated results and theoretical frameworks:
Theoretical models propose that under the regime of strong coupling, it is feasible to inhibit particular photochemical reactions by precisely manipulating the energy landscape. By adjusting the resonance of the cavity, one can modify the relative energy of the reactants and products by adjusting the cavity’s resonance, which has the potential to inhibit unwanted reactions.
Methods to Inhibit Photochemical Reactions:
Various approaches have been suggested to inhibit photochemical reactions in the regime of strong coupling:
Cavity Design: By intentionally designing cavities with appropriate resonances, it is possible to modify the energy distribution and effectively inhibit unwanted reactions.
Material Selection: Utilizing materials with precise qualities can amplify the robust coupling effect and expand the ability to manipulate reaction pathways.
The thermal disorder can be mitigated, and the uniformity of response outcomes can be improved by controlling the temperature.
Practical uses and illustrations in real-life situations:
Although there is potential in theory, practical implementations frequently expose the constraints imposed by thermal disorder. Thermal fluctuations have impeded efforts to improve performance in organic photovoltaic systems by utilizing strong coupling, as they interfere with the precise control necessary for maximum performance. For the same reason, in photocatalytic systems, thermal instability could make methods meant to stop unwanted reactions less effective. Measured and predicted excitation spectra of strongly coupled HBQ-cavities.
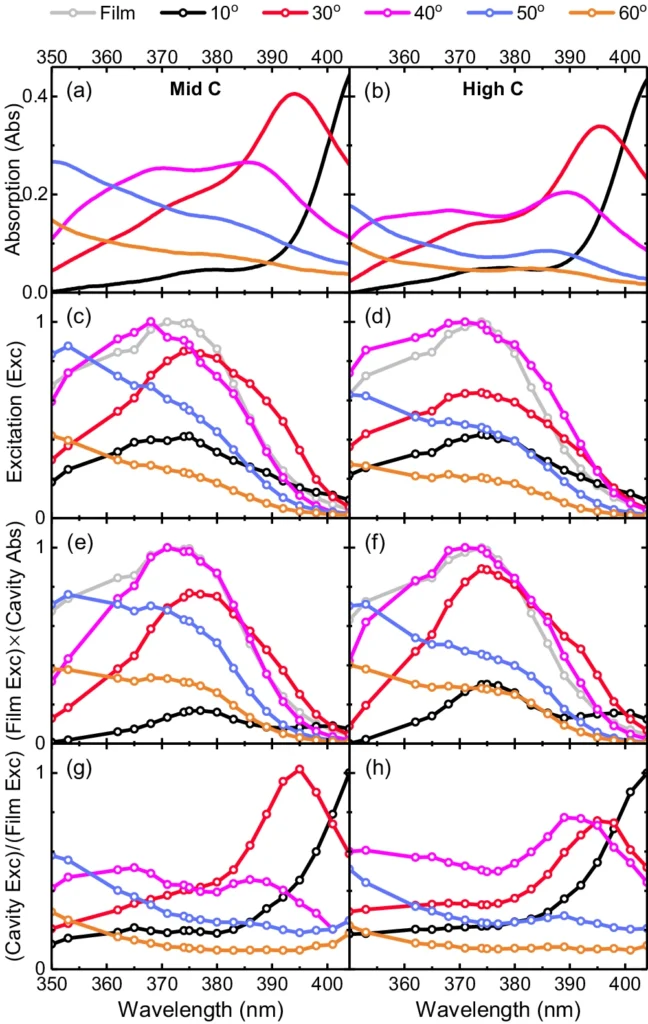
The inhibitory effect of thermal disorder:
The thermal disorder causes random changes in the energy levels and states of a molecular system, which makes it hard to do the exact thing that is needed to stop very fast photochemical reactions.
Understanding the underlying mechanisms:
We can conceptualize thermal fluctuations as unpredictable disturbances in the potential energy landscape of a molecular system. These disturbances can modify the relative energy of reactants, intermediates, and products, resulting in modifications to reaction rates and routes. Fluctuations in the strong coupling regime might hinder precise control over the energy landscape, thereby impeding the suppression of specific processes.
In-depth analysis of thermal fluctuations:
Thermal fluctuations are an intrinsic characteristic of molecular systems, resulting from the stochastic movement of molecules and atoms caused by thermal energy. With a temperature rise, the oscillations become more noticeable, resulting in a broader range of molecule locations and orientations. Because these reactions are naturally unpredictable, they can cause spectral lines to get wider, reaction speeds to change, and reaction pathways to change. This makes it hard to consistently control ultra-fast photochemical processes.
Studies that use analysis and simulation:
Studies utilizing analysis and computer simulations have yielded useful knowledge regarding the influence of thermal disorder on extremely rapid photochemical reactions. Molecular dynamics simulations have demonstrated that heat fluctuations can induce substantial alterations in the energy landscape of a reaction, resulting in modifications to reaction rates and pathways. In the same way, analytical models have shown that thermal disorder can make strategies for stopping certain responses less effective. This shows how hard it is to have precise control when temperatures are changing.
Recent Studies and Discoveries:
Current research has concentrated on comprehending the degree to which thermal disorder affects rapid photochemical reactions in the strong coupling regime. These findings emphasize the critical role that temperature and molecular dynamics play in influencing the possibility of controlling reactions.
Overview of Recent Research:
Recent research has investigated many facets of thermal disorder and its influence on rapid photochemical reactions. Researchers have examined the impact of temperature on reaction speeds and routes, discovering that higher thermal disorder can result in notable alterations in reaction dynamics. Previous studies have mostly looked at how molecular dynamics affects the effectiveness of strong coupling techniques, highlighting how hard it is to manipulate things accurately when there are temperature changes.
Important discoveries and their consequences:
The current research findings emphasize the substantial influence of thermal disorder on rapid photochemical reactions in the strong coupling regime. These discoveries have numerous significant ramifications:
The influence of thermal disorder highlights the difficulties in generating and sustaining strong coupling in actual systems.
Temperature control’s significance lies in its ability to manage thermal disturbance and improve the consistency of reaction outcomes within a system.
The need for advanced materials stems from the desire to improve the efficacy of strong coupling techniques and gain better control over ultra-fast photochemical processes by developing materials with lower thermal fluctuations.
An Overview of Various Research Methodologies:
Various research methodologies have offered further perspectives on the influence of thermal disorder on rapid photochemical reactions. The real-life challenges of keeping regulation stable when temperatures change have been revealed through experiments. On the other hand, analytical and simulation-based research has given a deep understanding of the basic mechanisms at play. Through the integration of these methodologies, scientists can cultivate a more all-encompassing comprehension of the ramifications of thermal disorder and discern tactics for alleviating its consequences.
Implications of Technology and Practical Applications:
The presence of thermal disorder poses a challenge in effectively controlling ultra-fast photochemistry, which has important ramifications for a wide range of technologies and practical applications.
Effects on Photonic Devices:
Controlling ultra-fast photochemical reactions is essential in photonic devices, such as organic photovoltaics and photonic circuits, to attain optimal performance and efficiency. Thermal disorder can diminish the efficacy of performance-enhancing techniques, resulting in decreased device efficiency and dependability.
The field of Material Science holds significant importance:
Mastery of photochemical processes is critical in the field of material science because it facilitates the development of sophisticated materials with precise and tailored features. Thermal disorder can interfere with the precise regulation required for these processes, making it difficult to achieve the correct material properties.
Prospects for Advancements in Photochemistry and the Interaction Between Light and Matter:
Gaining insight into the influence of thermal disorder on rapid photochemical reactions and the interaction between light and matter is crucial for the advancement of novel approaches and technologies. Subsequent investigations should prioritize the following areas:
Developing advanced materials entails creating substances that have lower thermal fluctuations. This can lead to improved effectiveness in strong coupling strategies and greater control over ultra-fast photochemical reactions.
Implementing cutting-edge cooling strategies can effectively reduce the impact of thermal disorder and enhance the consistency of reaction outcomes.
By integrating experimental, analytical, and simulation-based approaches, researchers can gain a more thorough understanding of the impacts of thermal disorder and develop effective strategies to minimize its influence. Measured emission yields as a function of coupling strength.
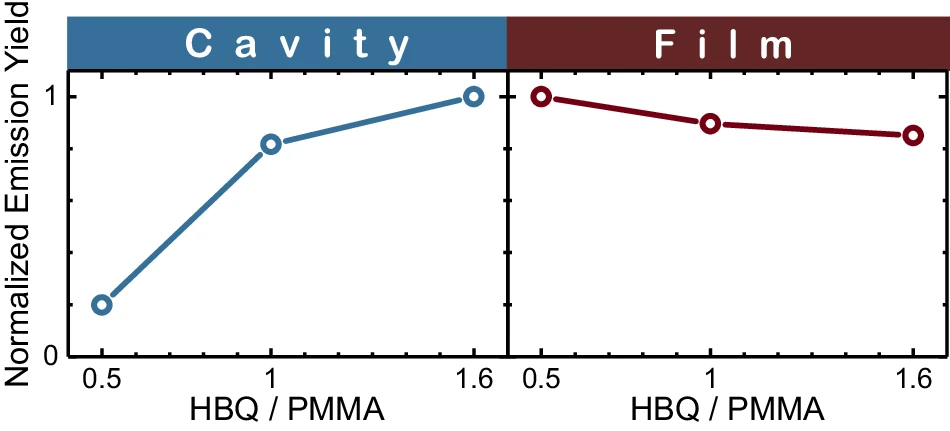
Potential remedies and areas for further investigation are identified:
Researchers are now investigating different tactics and potential future research directions to reduce the impact of thermal disorder and improve control over ultra-fast photochemical processes.
Methods to Alleviate Thermal Disorder:
To lessen the effects of thermal disorder, you can use a variety of measures:
Advanced Materials: The development of materials with reduced thermal fluctuations can boost the efficacy of strong coupling techniques and provide better control over ultra-fast photochemical reactions.
Utilizing sophisticated cooling strategies can effectively mitigate temperature variations and enhance the uniformity of reaction results.
Environmental control involves the regulation of environmental variables, such as temperature and pressure, to reduce the impact of thermal disorder and improve the management of photochemical reactions.
Advanced materials and cooling techniques:
It is critical to develop sophisticated materials and cooling systems to reduce the impact of thermal disorder. For example, materials with lower thermal conductivity can help to reduce thermal fluctuations. Additionally, we can employ advanced cooling methods like cryogenic cooling to sustain the precise conditions necessary for achieving strong coupling.
Potential Areas for Future Research:
Subsequent investigations should prioritize the following areas:
Gaining a comprehensive understanding of the mechanisms that cause thermal disorder and how it affects ultra-fast photochemistry is crucial for identifying effective solutions to minimize its influence.
Novel materials with reduced thermal fluctuations and improved characteristics can boost the efficacy of strong coupling techniques and provide greater control over ultra-fast photochemical reactions.
Researching novel methodologies: Examining innovative ways and strategies for regulating a system’s temperature and atmosphere can help reduce the impact of thermal irregularities and improve the uniformity of response results.
In conclusion:
The presence of thermal disorder is a significant obstacle to effectively inhibiting rapid photochemical reactions in the regime of strong interaction between light and matter. The unpredictable effects of thermal fluctuations often hinder the actual implementation of strong coupling, despite its great theoretical potential for regulating photochemical reactions. Gaining insight into the consequences of thermal disorder and devising methods to minimize its influence is crucial for fully exploiting the significant interactions between light and matter.
Frequently Asked Questions:
1). What is the term for ultra-fast photochemistry?
Ultra-fast photochemistry refers to chemical reactions that take place within extremely brief time intervals, ranging from femtoseconds (10-15 seconds) to picoseconds (10-12 seconds). These reactions play a critical role in activities such as photosynthesis and solar energy production.
2). What is the mechanism behind light-matter coupling?
Light-matter coupling refers to the interaction that occurs between photons (light) and atoms or molecules (matter). Strong coupling refers to a contact that is highly intense, resulting in the formation of hybrid states known as polaritons, which possess distinct characteristics.
3). Thermal disorder refers to the state of randomness or lack of order in the distribution of thermal energy inside a system.
Thermal disorder is the result of thermal energy’s stochastic movement of molecules and atoms in a system. It exhibits a positive correlation with temperature and has the potential to disturb structured molecular configurations.
4). What makes it difficult to control photochemical reactions in the strong coupling regime?
Although strong coupling has the potential to regulate photochemical reactions, the presence of thermal disorder provides unpredictable variations that hinder this regulation, making it difficult to inhibit.
5). What are possible strategies to reduce the impact of thermal disorder?
Possible solutions include using modern materials with reduced thermal fluctuations, employing novel cooling strategies, and conducting additional research to improve our understanding and regulation of the interaction between thermal energy and strong coupling.
For more chemistry blogs, visit chemistry Master