Table of Contents
Overview of Triplet Carbenes :
Triplet carbenes, a distinctive category of reactive intermediates in the field of organic chemistry, have captivated scientists for many years because of their peculiar electrical structure and exceptional reactivity. These compounds have a divalent carbon atom with two unpaired electrons in different orbitals, as well as a ground state in a triplet configuration. The unusual features of these structures, resulting from their electrical configuration, are not only theoretically fascinating but also very relevant in a wide range of chemical reactions and processes.
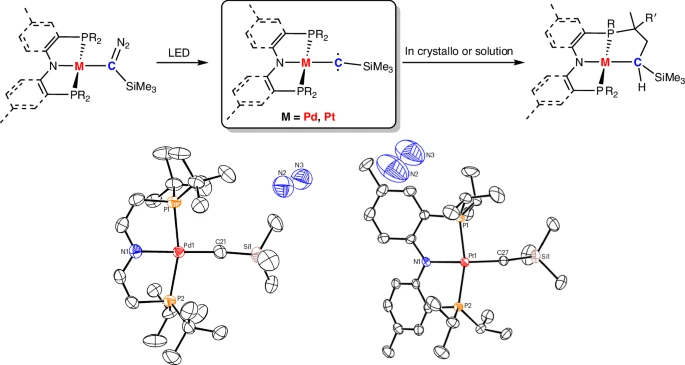
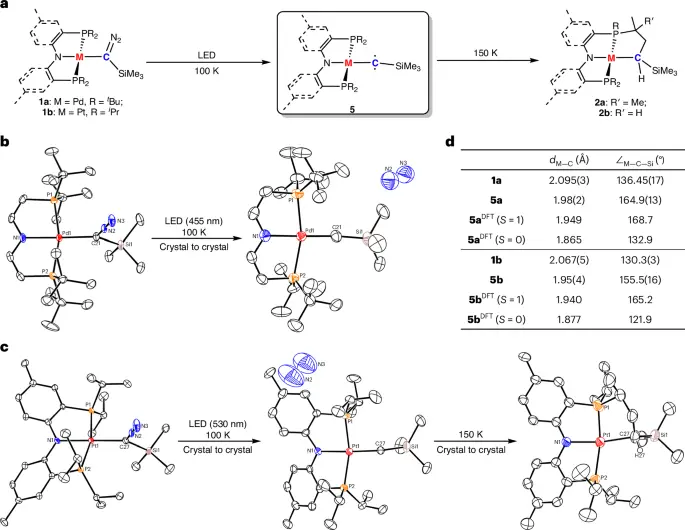
Adding transition-metal substituents to triplet carbenes has greatly increased their usefulness by making them more stable and giving them new ways to react. An important part of making carbenes more useful in synthetic chemistry, catalysis, and materials science is transition metals, which are known for their flexible coordination chemistry and ability to stabilize reactive intermediates.
An Exploration into Triplet Carbenes:
Fundamental Framework and Electron Arrangement:
Triplet carbenes have two unpaired electrons in degenerate orbitals, resulting in a paramagnetic ground state. In contrast to singlet carbenes, this configuration involves the pairing of two electrons in one orbital, leading to a diamagnetic ground state. The triplet state is generally more stable because it experiences less electron-electron repulsion.
Contrast with Singlet Carbenes:
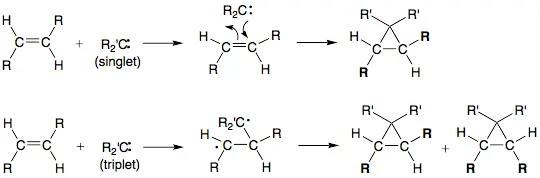
Although singlet and triplet carbenes both have a divalent carbon atom, their electronic configurations result in distinct reactivities. Singlet carbenes, which contain a pair of electrons, frequently participate in electrophilic or nucleophilic processes, depending on the substituents connected to the carbene’s carbon atom. Conversely, triplet carbenes generally engage in radical-type reactions because of their unpaired electrons.
Generation Techniques:
In photolytic methods, light is used to break chemical bonds in precursor molecules, like diazo compounds, so that triplet carbenes can be made. This technique is beneficial for generating carbenes with high energy levels that can promptly interact with transition-metal complexes.
Thermal techniques utilize heat to trigger the decomposition of carbene precursors. This method often provides a controlled way to produce carbenes, which transition-metal coordination can stabilize.
Chemical approaches utilize reagents to transform precursors into triplet carbenes. For instance, the immediate formation of metal-carbene species occurs when diazo compounds combine with transition-metal complexes.
The role of transition metal substituents:
Transition metals play a significant role in organometallic chemistry:
Transition metals play a crucial role in organometallic chemistry because they can create stable complexes with various ligands, such as carbenes. Because they have unique electrical properties, such as changing oxidation states and the ability to donate electrons back to the atom, transition metals are great for stabilizing reactive intermediates like carbenes.
Transition metals are frequently used:
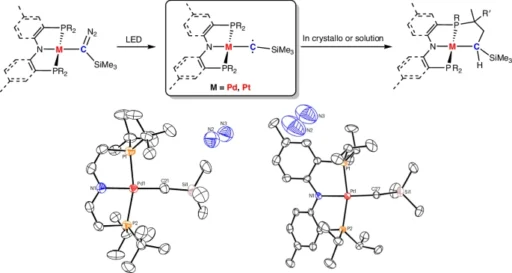
Rhodium: Carbene chemistry extensively uses rhodium because it can form stable complexes and facilitate a wide range of catalytic processes.
Palladium is a valuable metal because of its ability to stabilize carbene and facilitate synthetic transformations, according to its versatility in cross-coupling reactions.
Platinum is frequently used to create durable carbene complexes due to its stability and strong metal-ligand interactions.
Other transition metals, such as ruthenium, iridium, and gold, also have important functions in carbene chemistry. Each of these metals has distinct benefits in reactivity and stability.
Synthesis of Triplet Carbenes with Transition-Metal Substituents:
Principles for Creating Synthetic Compounds:
Coordination chemistry offers a systematic approach to creating and producing metal-carbene complexes. Chemists can manipulate the stability and reactivity of carbene.
The selection of ligands is pivotal in the synthesis of carbenes. Ligands that provide steric shielding and electrical stabilization to the carbene center can significantly improve the complex’s stability.
Concrete illustrations and systematic approaches:
Rhodium-carbene complexes: Diazo compounds serve as precursors in the synthesis of rhodium-carbene complexes. Rhodium complexes facilitate the breakdown of diazo compounds, creating stable rhodium-carbene species. These species exhibit a high level of reactivity and are effective catalysts in several chemical reactions.
Palladium-carbene complexes: Reacting palladium precursors with carbene ligands commonly forms palladium-carbene complexes. These compounds are renowned for their efficacy in cross-coupling reactions and other catalytic activities.
Platinum-carbene complexes: Similar procedures used for rhodium and palladium can also produce platinum-carbene complexes. Platinum’s stability enables the creation of strong carbene complexes, which have practical uses in synthetic and material science fields.
Stability and reactivity:
Factors affecting stability:
Electronic Effect: The electronic environment surrounding the carbene center greatly influences its stability. Through a process called “back-donation,” transition-metal substituents can add electron density to the carbene, which makes it more stable.
Steric effects refer to the phenomenon in which bulky ligands protect the carbon core, preventing it from interacting with reactive species and increasing its stability.
Patterns of reactivity:
Addition Reactions: Triplet carbenes can undergo addition reactions with various types of multiple bonds, including alkenes and alkynes. This leads to the formation of additional products, such as cyclopropanation and other similar compounds.
During insertion reactions, triplet carbenes are added to C-H and C-C bonds. This usually leads to the formation of new bonds between carbon atoms or between carbon atoms and heteroatoms.
Cycloadditions: Triplet carbenes engage in cycloaddition processes, generating ring structures that hold significant value in chemical synthesis.
Electron configuration and chemical bonding:
Summary of Electronic Configurations:
Triplet carbenes possess an electrical configuration characterized by two unpaired electrons occupying degenerate orbitals. The electronic arrangement of the metal and the presence of d-orbitals, which can facilitate back-donation, determine the interaction between the metal and carbon.
Interactions between metal and carbene compounds:
By engaging in back-donation, transition metals stabilize carbenes by transferring electron density from the metal’s d-orbitals to the carbene’s vacant p-orbital. This interaction serves to both stabilize the carbene and alter its reactivity.
Computational Studies and Theoretical Models: Computational studies refer to the use of computer simulations and mathematical algorithms are used in computational studies to investigate and analyze complex systems or phenomena. On the other hand, we use theoretical models, which are mathematical or conceptual frameworks, to explain and predict the behavior of these systems or phenomena.
Density Functional Theory (DFT) studies give a full picture of the electronic structure and behavior of metal-carbene complexes. Computational models aid in forecasting the stability and reactivity of novel carbene molecules.
Molecular orbital theory provides a theoretical framework for comprehending the bonding interactions in metal-carbene complexes. Chemists can explain the stability and reactivity of these complexes by studying the intersection of metal d-orbitals and carbene p-orbitals.
Spectroscopic characterization:
Methods Employed in Characterization:
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy reveals details on the electronic surroundings of the carbene carbon and the ligands attached to it. The chemical shifts observed in the NMR spectra can provide information about the carbene’s existence and characteristics.
We use infrared (IR) spectroscopy to identify functional groups and bond vibrations in metal-carbene complexes. The identification of specific carbene stretching modes can serve as evidence for the complex’s formation.
UV-Vis Spectroscopy: We employ UV-Vis spectroscopy to examine the electronic transitions occurring in metal-carbene complexes. These transitions convey information regarding the electrical configuration and bonding interactions.
Analyzing Spectroscopic Data:
Chemical shifts in NMR spectra provide information about the carbon-carbon’s electronic surroundings. Downfield changes imply the presence of electron-withdrawing processes, while upfield shifts show the occurrence of electron-donating interactions.
Spectra that look for vibrational modes in the infrared range (IR) can tell you a lot about how metal-carbene complexes are bonded. Distinctive stretching frequencies can verify the existence of particular bonds and functional groups.
By displaying electronic transitions, UV-Vis spectra provide insight into the electrical structure of metal-carbene complexes. These transitions aid in understanding the essence of metal-to-carbene back-donation and the complex’s general stability.
Organic synthesis applications:
Function in Catalytic Processes:
Cross-coupling processes rely on triplet carbenes with transition-metal substituents. These carbenes are very efficient in forming carbon-carbon and carbon-heteroatom bonds. These reactions play a crucial role in organic synthesis, offering a flexible method for building intricate structures.
Cyclopropanation is the process of adding triplet carbenes to alkenes, which leads to the creation of cyclopropane rings. This reaction is highly advantageous for producing strained ring structures and has extensive utility in the synthesis of natural goods and medicines.
C-H Activation: Triplet carbenes enable the direct modification of unactivated C-H bonds through a process known as C-H activation. The reactivity is particularly desirable in organic synthesis due to its capacity to incorporate functional groups into otherwise unreactive locations.
Synthetic Transformations Enabled by Carbenes
Formation of Complex Molecules: The distinctive reactivity of triplet carbenes enables the synthesis of complex compounds with high precision. Because they can participate in a variety of addition and insertion processes, they serve as important intermediates in multi-step synthetic routes.
Stereoselectivity and Regioselectivity: Triplet carbenes frequently demonstrate significant stereoselectivity and regioselectivity throughout their reactions. The ability to selectively synthesize chiral compounds and regioisomers is of utmost importance in the production of medicines and agrochemicals.
The field of Material Science has numerous applications:
Application in Developing Sophisticated Materials:
Organic light-emitting diodes (OLEDs) employ triplet carbenes containing transition-metal substituents for advancement. Due to their exceptional photophysical characteristics, such as elevated luminescence and stability, they are highly suitable for the development of efficient light-emitting materials.
Solar Cells: Triplet Carbene’s distinctive electrical characteristics contribute to the advancement of solar cells. These materials possess a high capacity to absorb light and effectively transform it into electrical energy, rendering them highly beneficial for solar energy applications.
Optical characteristics:
Luminescence: Many applications, including sensors and display technology, utilize the light-emitting characteristics of triplet carbenes with transition-metal substituents. In many applications, their ability to produce light when stimulated is critical.
Charge Transport: Triplet carbenes can facilitate charge transmission in organic electrical devices. Because of their ability to stabilize charges and engage in electron transfer processes, they are highly useful constituents in electronic materials.
Biological Consequences:
Possible applications in the field of biochemistry are possible:
Triplet carbenes have potential applications in biochemistry, specifically as tools for investigating and analyzing biological processes. We can utilize their distinctive reactivity to mark biomolecules or to trigger precise chemical changes in biological systems.
Assessing the toxicity and ensuring safety:
Due to their extreme reactivity, triplet carbenes require cautious handling to prevent unwanted side reactions and toxicity. Thorough safety assessments are required to guarantee the safe use of these substances in biological and medicinal contexts.
Applications of Medicinal Chemistry:
Triplet carbenes serve as intermediates in the production of bioactive molecules in medical chemistry. Their ability to construct intricate molecular configurations makes them invaluable tools for developing novel pharmaceuticals and therapeutic agents.
Obstacles and Constraints:
Creating artificial substances presents challenges:
Because they are naturally reactive, it can be hard to make stable triplet carbenes with transition metal substituents. It is critical to develop strong and reliable synthetic techniques to overcome these challenges and consistently manufacture these chemicals.
Stability concerns:
The stability of triplet carbenes is a crucial determinant in their utilization. While the introduction of transition-metal substituents can enhance stability, maintaining this stability under various conditions remains a challenging task.
Real-world implementations and scalability:
The process of increasing the production of triplet carbenes for industrial use has practical difficulties. In large-scale production, it is necessary to optimize synthetic procedures and meticulously manage reaction conditions.



Recent Advances and Research Trends:
State-of-the-art investigation:
The development of innovative synthesis techniques and the exploration of unexplored applications have primarily driven the latest progress in the domain of triplet carbenes featuring transition-metal substituents. Advances in ligand design and metal coordination have broadened the scope of these compounds.
The development of artificial techniques has advanced significantly:
We have devised novel synthetic techniques to enhance the productivity and robustness of triplet carbenes. These strategies involve the utilization of innovative starting materials, sophisticated photolytic and thermal procedures, and the implementation of computational models to direct the synthesis process.
Prospects for Advancements in the Field:
We expect subsequent investigations to explore novel applications of triplet carbenes in fields such as catalysis, materials science, and medicinal chemistry. The field will experience further breakthroughs through interdisciplinary cooperation and the integration of computational and experimental methodologies.
Case Studies:
The role of Rhodium-Carbene Complex in Catalysis:
A case study highlights the effectiveness of rhodium-carbene complexes in catalytic processes. Cyclopropanation processes utilize rhodium-carbenes, yielding significant amounts of product with excellent specificity. Their ability to promote the creation of tense ring structures makes them valuable assets in synthetic chemistry.
The field of material science employs platinum-carbene complexes:
Researchers have thoroughly investigated the stability and reactivity of platinum-carbene complexes. Organic electronic materials like OLEDs and solar cells utilize these compounds in their fabrication. Due to their strong and distinctive electrical properties, they are well-suited for these specific applications.
The use of palladium-carbene complexes in organic synthesis is being explored:
Palladium-carbene complexes are very important in cross-coupling reactions because they make it easier for carbon atoms to bond with each other and with heteroatoms. A case study illustrates the plasticity of palladium-carbene complexes in synthetic transformations and their significance in contemporary organic synthesis.
Prospects for the Future:
Possible Novel Applications:
The potential applications of triplet carbenes with transition-metal substituents are growing. Compounds like sustainable chemistry, green catalysis, and advanced materials science present fresh prospects in emerging domains.
Opportunities for Interdisciplinary Research:
The integration of chemistry, physics, materials science, and biology in interdisciplinary research will propel the advancement of novel applications for triplet carbenes. Collective efforts can lead to significant advancements in understanding and harnessing these distinct intermediaries.
Effect on Sustainable Chemistry:
Triplet carbenes with transition-metal substituents can enhance sustainable chemistry by facilitating more effective and specific catalytic reactions. Applying them in the field of green chemistry can contribute to waste reduction and enhance the overall sustainability of chemical manufacturing.
In conclusion:
Triplet carbenes that have transition-metal substituents are an intriguing and quickly developing field of chemistry. Due to their distinctive electronic features and wide range of uses, they are highly valuable instruments in both academic research and industry settings. Further investigation and advancement in this domain hold the potential to uncover untapped possibilities, resulting in notable progress in the areas of synthesis, catalysis, and materials science.
Frequently Asked Questions:
1). What are triplet carbenes?
In organic chemistry, triplet carbenes are very reactive compounds made up of a carbon atom with two free electrons in orbitals with the same amount of energy. This makes the compound paramagnetic.
2). Carbenes use transition metals because they can stabilize and facilitate the reactivity of these highly reactive species.
Carbenes use transition metals to stabilize highly reactive intermediates and provide distinctive reactivity. Their capacity to participate in back-donation and exhibit changing oxidation states renders them well-suited for altering carbene characteristics.
3). What is the method of synthesizing triplet carbenes?
Photolytic, thermal, and chemical techniques can produce triplet carbenes, typically by decomposing diazo compounds or reacting carbene precursors with transition metal complexes.
4). What are the uses of triplet carbenes?
Triplet carbenes find use in organic synthesis, catalysis, and material research. They have significant functions in catalytic processes, the advancement of sophisticated materials like OLEDs and solar cells, and potentially in biochemistry and pharmaceutical chemistry.
5). What challenges does this specific domain present?
The field of triplet carbenes faces challenges such as synthetic complexity, stability concerns, and the ability to produce them on a larger scale for practical uses. To tackle these difficulties, it is critical to develop strong and effective synthetic approaches.
For more chemistry blogs, visit chemistry Master