Table of Contents
Overview of Veratrum Alkaloids:
Veratrum alkaloids are a class of natural compounds obtained from plants in the genus Veratrum. They are known for their intricate structures and notable biological efficacy. These molecules possess a steroidal framework fused with distinctive structural motifs, rendering them significant targets for synthetic chemists. Traditional medicine has used these alkaloids, predominantly found in species like Veratrum album and Veratrum viride, to treat conditions such as hypertension, inflammation, and discomfort. Recently, substances like cyclopamine, a Veratrum alkaloid, have attracted interest for their capacity to block the Hedgehog signaling pathway, a crucial factor in specific cancer types.
The synthesis of Veratrum alkaloids is challenging due to their biological significance and complex molecular structure. Because they have a rigid polycyclic framework, many stereocenters, and a lot of functionalization, they are some of the hardest compounds to make in a lab. Divergent synthesis is a method that enables chemists to derive many complex compounds from a shared precursor, thereby optimizing the synthetic process and enhancing efficiency. This article examines the varied syntheses of complicated Veratrum alkaloids, focusing on both historical and contemporary methodologies for their creation. Reported syntheses of Veratrum alkaloids.
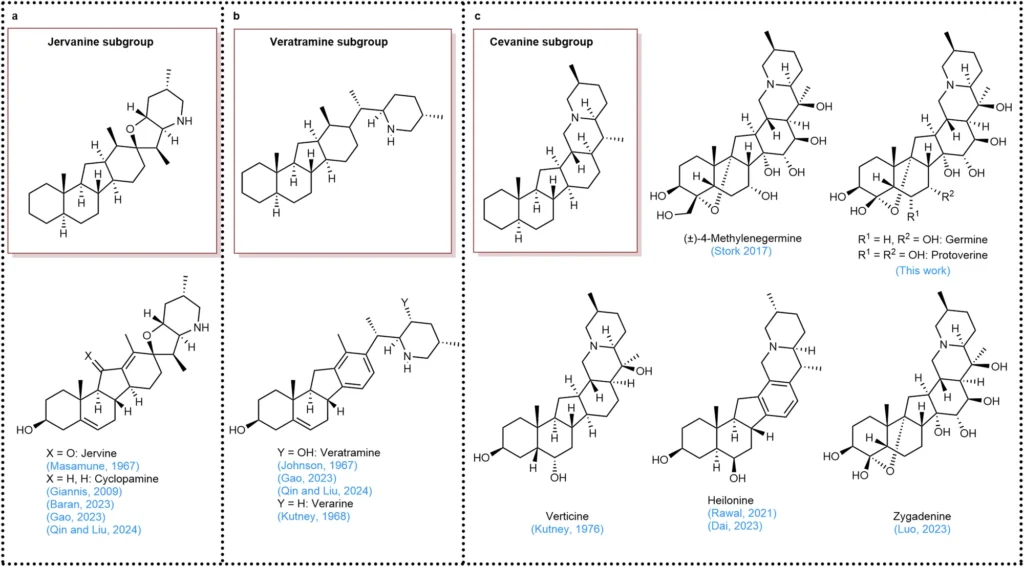
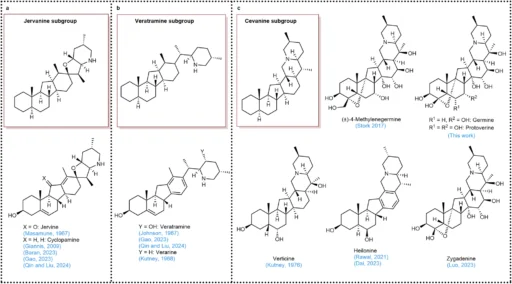
photo credit a Framework of the jervanine subgroup. b Framework of the veratramine subgroup. c Framework of the cevanine subgroup.
Veratrum alkaloids’ structural complexity:
Veratrum alkaloids are complicated chemicals with a steroidal backbone that is made up of joined six-membered rings in a polycyclic structure. Many functional groups embellish this inflexible structure, allowing for substantial modification of the alkaloid’s biological activity. Small changes to the stereochemistry or functionalization of the core can lead to compounds that have very different drug-like properties.
Characterizing the Structural Attributes of Veratrum Alkaloids:
Steroidal Core: The majority of Veratrum alkaloids possess a shared steroidal framework, comprising three six-membered rings (designated A, B, and C) and one five-membered ring (designated D). This tetracyclic structure provides the structural stiffness necessary for these chemicals to interact with biological targets.
Stereocenters: The stereochemistry of Veratrum alkaloids is essential for their biological action. During synthesis, one must meticulously regulate the several stereocenters these compounds frequently possess. Any alteration from the native conformation may lead to a loss of activity or the formation of a completely distinct bioactive molecule.
Functional Groups: Hydroxyl groups, amines, and other moieties frequently modify the fundamental structure of Veratrum alkaloids to enhance their biological activity. Synthetic chemistry presents a considerable difficulty in selectively and precisely introducing these functional groups.
The synthesis of Veratrum Alkaloids faces obstacles:
The intricate structure of Veratrum alkaloids renders their synthesis particularly challenging. The principal challenges consist of:
Stereochemical Regulation: Maintaining the appropriate stereochemistry at each stereocenter is essential to alkaloid synthesis. The abundance of stereocenters in Veratrum alkaloids makes this a formidable challenge.
Functional Group Tolerance: We must execute the incorporation of functional groups, such as hydroxyls and amines, in a manner that does not disrupt the integrity of the remaining molecular structure. This frequently necessitates the utilization of protective groups and selective responses.
It’s hard to make the needed carbon-carbon bonds with high selectivity because the Veratrum alkaloids have a stiff polycyclic structure.
An Overview of Divergent Synthesis:
In organic synthesis, two principal methodologies for creating complex compounds are convergent synthesis and divergent synthesis. Divergent synthesis involves building distinct molecular components separately and then combining them in the final stages. On the other hand, divergent synthesis starts with a shared intermediate and diverges to produce many unique products. Divergent synthesis is a good way to get different Veratrum alkaloids from a common starting material. These alkaloids are from the same structural family.
What constitutes divergent synthesis?
Divergent synthesis is a methodology that facilitates the generation of several products from a singular starting ingredient or intermediate. This method is especially advantageous for addressing a family of related natural compounds, as it allows scientists to produce structural diversity from a shared precursor. Divergent synthesis depends on the ability to execute selective reactions on the intermediate, resulting in a large number of final products.
Divergent synthesis is an appealing approach in the context of Veratrum alkaloids because many of them share a common core structure. By discovering a shared intermediate, chemists can obtain many Veratrum alkaloids with minor differences in functional groups or stereochemistry.
Benefits of Divergent Synthesis in Complex Alkaloids Construction:
Increased Efficiency: Divergent synthesis is more efficient than linear synthesis because it lets chemists make more complex alkaloids from a single starting material in fewer steps.
Flexibility: Divergent synthesis lets you change functional groups and control stereochemistry, which makes it easier to target different members of the alkaloid family.
Resource Conservation: Divergent synthesis makes it possible to make many products from a single intermediate, which reduces the amount of reagents and starting materials that need to be used and also makes the process more cost-effective.
Time-Saving: The ability to synthesize several compounds from a common precursor significantly reduces the total time required to produce intricate natural products. Retrosynthetic analysis of complex Veratrum alkaloids.
Historical Methods for Synthesizing Veratrum Alkaloids:
The synthesis of Veratrum alkaloids has a lengthy history, with first efforts depending on linear synthetic methodologies. These traditional methods involved incremental assembly of the alkaloid structure, frequently necessitating numerous discrete reactions to form the intricate polycyclic framework. Although these approaches effectively generated limited amounts of Veratrum alkaloids, they were markedly inefficient and resource-demanding.
Traditional synthetic approaches:
The first attempts to make Veratrum alkaloids focused on copying the biosynthetic pathways seen in nature. This often entailed synthesizing the steroidal core initially and subsequently modifying it to produce the desired alkaloid. These traditional tactics were often linear, with each phase constructing upon the preceding one.
One example is the production of veratramine, a significant Veratrum alkaloid. Initial veratramine syntheses entailed the assembly of the steroidal framework via a sequence of cyclization processes, followed by functionalization to incorporate the requisite hydroxyl and amine groups. Although these endeavors successfully yielded modest amounts of veratramine, the entire procedure was protracted and inefficient, frequently necessitating 20 or more stages.
Conventional approaches have limitations:
Elevated Step Count: Traditional synthetic methodologies frequently included numerous discrete steps, each of which presented opportunities for yield reduction and adverse reactions.
Suboptimal Yields: The intricate multi-step processes inherent in previous syntheses of Veratrum alkaloids frequently resulted in decreased overall yields, thereby complicating the production of substantial quantities of these chemicals.
Inadequate Stereochemical Regulation: Regulating the stereochemistry of numerous stereocenters poses a considerable challenge in traditional synthesis. Initial techniques frequently failed to provide the stereochemical accuracy necessary for physiologically active Veratrum alkaloids.
Resource-Intensive: The linear approach of conventional synthesis necessitated substantial amounts of reagents and starting materials, escalating prices and diminishing sustainability.
Recent Developments in Divergent Syntheses of Veratrum Alkaloids:
Recent advancements in organic chemistry have resulted in the creation of more efficient and selective methodologies for synthesizing complicated natural compounds, such as Veratrum alkaloids. In these breakthroughs, divergent synthesis has been essential, enabling chemists to obtain various Veratrum alkaloids from the same precursor.
Innovative Approaches in Contemporary Organic Chemistry:
Transition Metal Catalysis: Transition metals, such as palladium, rhodium, and ruthenium, have changed organic synthesis by making it easier to form very specific bonds between carbon atoms. These metals can facilitate reactions that are challenging or unattainable by conventional methods, making them essential instruments for the synthesis of intricate alkaloids.
Organocatalysis: Organocatalysis, utilizing tiny organic molecules as catalysts, has emerged as a potent method for synthesizing Veratrum alkaloids. Organocatalysts are perfect for making delicate natural compounds because they are very selective and the reaction conditions are very mild.
Asymmetric Catalysis: Regulating stereochemistry is a primary problem in the synthesis of Veratrum alkaloids. Asymmetric catalysis uses chiral catalysts to achieve antiselectivity, which solves the problem by making it easier to make only the required stereoisomers.
Cascade Reactions: Cascade reactions, characterized by several bond-forming processes occurring simultaneously, have demonstrated utility in the synthesis of intricate alkaloids. These reactions facilitate the rapid assembly of polycyclic frameworks, hence minimizing the total number of steps necessary for synthesis. Evolution of strategies to functionalize the Δ6,7-alkene.
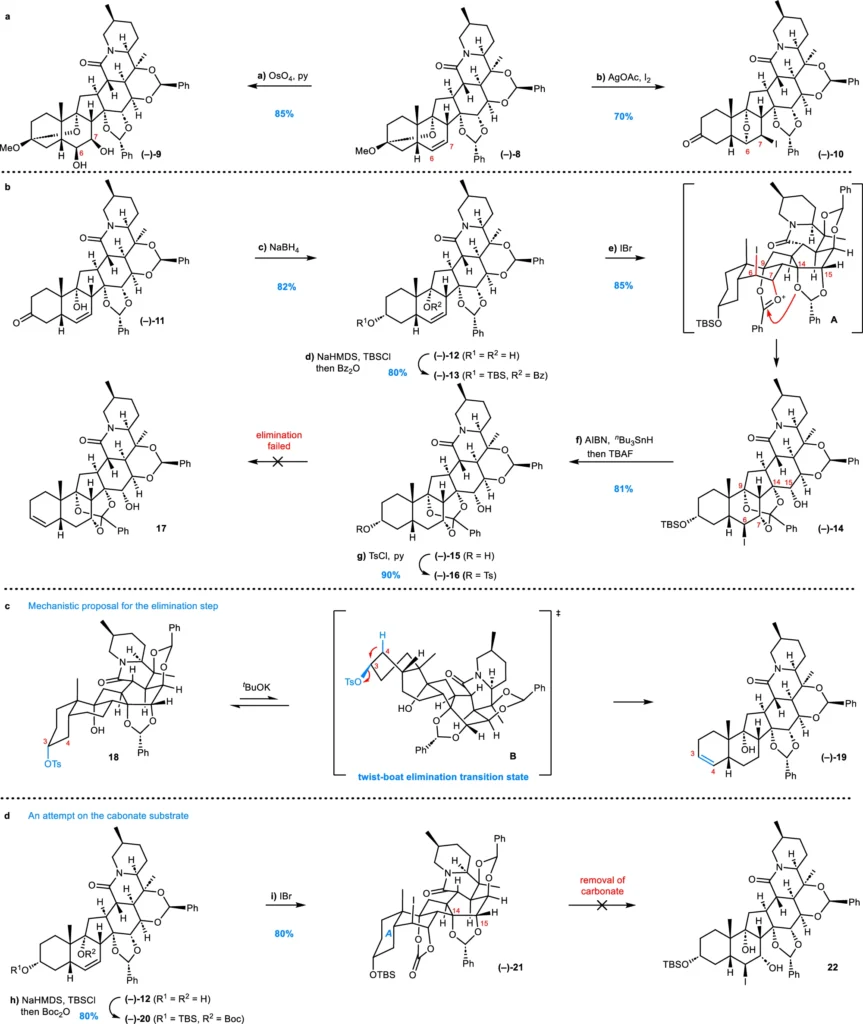
Case Studies of Effective Divergent Synthesis:
Synthesis of Veratramine:
A divergent strategy, starting with a shared polycyclic intermediate, synthesizes veratramine, a principal Veratrum alkaloid. Scientists can selectively modify this intermediate, which features the fundamental steroidal structure, to produce veratramine or other analogous alkaloids. Chemical scientists can make veratramine in fewer steps than usual by changing certain functional groups, such as by hydroxylation and amination.
Synthesis of Cyclopamine:
Similar, diverse techniques have produced cyclopamine, a complex Veratrum alkaloid. Chemists have used a sequence of selective bond-forming processes to synthesize the polycyclic structure of cyclopamine from a shared steroidal source. The essence of this synthesis is the meticulous regulation of stereochemistry and the strategic incorporation of functional groups at specific locations throughout the synthetic route.
Principal Reactions Utilized in Divergent Synthesis:
The diverse synthesis of Veratrum alkaloids utilizes numerous essential reactions. These reactions facilitate the selective synthesis of carbon-carbon and carbon-heteroatom bonds, allowing the polycyclic framework to be assembled and the molecule to be functionalized.
The formation of carbon-carbon bonds involves several reactions:
Aldol Reactions: The Aldol reaction is an effective method for constructing carbon-carbon bonds in organic synthesis. Veratrum alkaloids frequently employ aldol reactions to establish the essential carbon-carbon bonds connecting the steroidal rings.
Michael Addition: By letting a nucleophile add to an α,β-unsaturated carbonyl molecule, Michael addition reactions make it easier to make carbon-carbon bonds. Divergent synthesis commonly uses this reaction to form the polycyclic structure of Veratrum alkaloids.
Cross-Coupling Reactions: Cross-coupling reactions sped up by transition metals, like Suzuki and Stille couplings, are needed to selectively form carbon-carbon bonds. Veratrum alkaloids frequently employ these reactions to connect their different constituents.
Essential cyclization reactions:
Ring-Closing Metathesis (RCM) is an effective technique for synthesizing cyclic structures from linear precursors. Ring-closing metathesis (RCM) frequently constructs the polycyclic steroidal framework in the synthesis of Veratrum alkaloids.
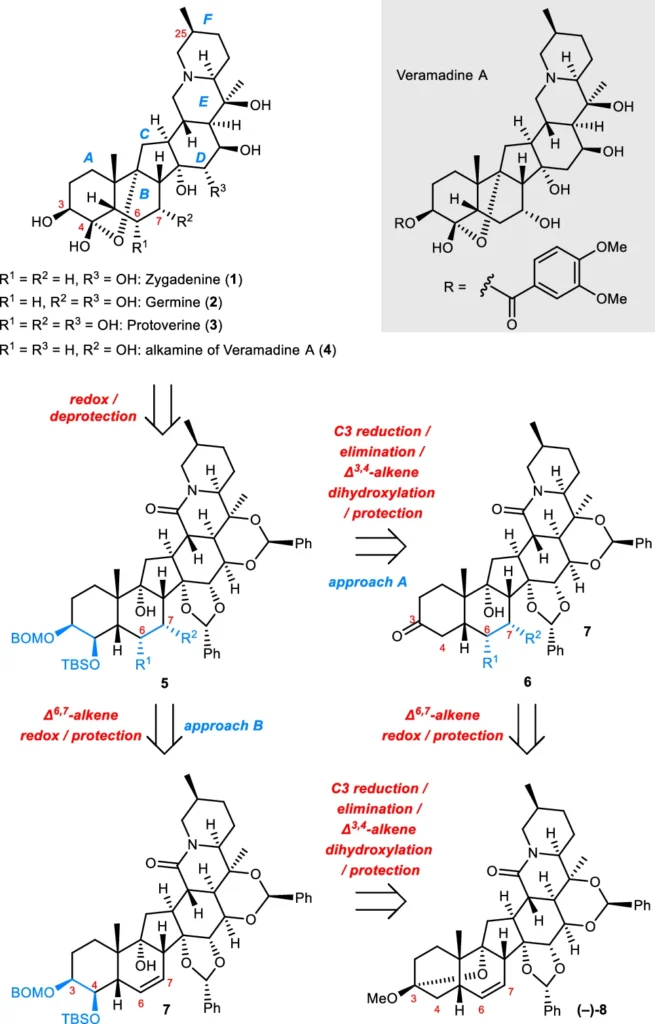
Intramolecular Cycloadditions: We employ cycloddition processes, where two unsaturated molecules combine to form a ring, to synthesize the polycyclic structure of Veratrum alkaloids. These reactions exhibit exceptional selectivity, facilitating the production of intricate cyclic structures in a singular step. Formal synthesis of (–)-zygadenine (1) and synthesis of the alkamine of veramadine A (–)-4.
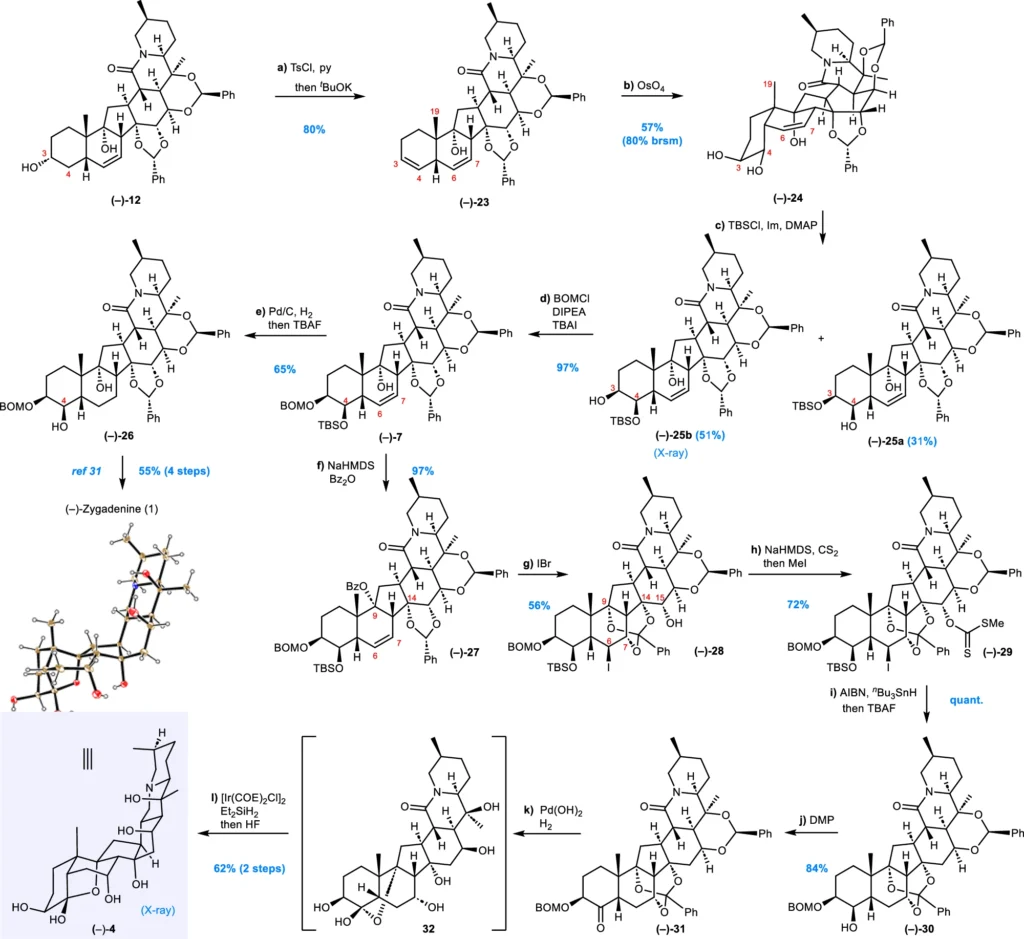
The Function of Catalysis in Divergent Syntheses:
Catalysis is essential in the varied synthesis of Veratrum alkaloids. Chemicals can facilitate reactions that might otherwise be inefficient or unselective by employing catalytic quantities of reagents. Both metal-catalyzed processes and organocatalysis have demonstrated their significance in the production of these intricate compounds.
The synthesis of alkaloids involves metal-catalyzed reactions:
The synthesis of Veratrum alkaloids commonly employs transition metals like palladium, rhodium, and ruthenium as catalysts. These metals can help with many processes, such as cross-coupling, hydrogenation, and cyclization. These are all very important for making the polycyclic structure of alkaloids.
Scientists frequently employ palladium-catalyzed cross-coupling processes to establish the carbon-carbon bonds that connect the steroidal rings. These reactions exhibit remarkable selectivity, enabling scientists to create the intended bonds without generating undesirable byproducts.
Organocatalysis in Veratrum Alkaloid Synthesis:
The manufacture of Veratrum alkaloids has applied organocatalysis, which uses tiny organic molecules as catalysts. Organocatalysts provide numerous benefits compared to metal catalysts, such as more moderate reaction conditions and enhanced selectivity. This renders them especially advantageous for reactions involving delicate functional groups or stereocenters.
One way that organocatalysis is used to make Veratrum alkaloids is by using proline as a catalyst in aldol reactions. Proline can cause a lot of enantioselectivity, which makes it easier to make the desired stereoisomers with few unwanted side effects.
Designing Synthetic Pathways for Multiple Alkaloid Targets:
The key to successful divergent synthesis is coming up with synthetic routes that can make more than one alkaloid from a single starting material. This necessitates meticulous planning and a profound comprehension of the intermediate’s responsiveness.
Modular Methodology for Divergent Synthesis:
The modular approach is a concept for constructing synthetic pathways that uses diverse building components to facilitate subsequent alterations. This method is especially advantageous for targeting a family of related alkaloids, as it allows for late-stage modifications to the molecular structure.
Various positions can modify a typical polycyclic intermediate to produce distinct Veratrum alkaloids. Chemists can generate various products from a single intermediate by altering the sequence of functional group installation or employing different reagents.
Standard Intermediate Strategy:
An alternative method selectively alters a shared intermediate to produce various end products. This method is very efficacious for synthesizing families of natural compounds, such as Veratrum alkaloids, that possess a shared core structure.
In the case of Veratrum alkaloids, the steroidal framework acts as a shared intermediate. By selectively modifying this core, chemists can generate various alkaloids with minimal alterations to the overall synthetic route.
Chemoselectivity and Stereoselectivity in Divergent Synthesis:
It is important to have high levels of chemo- and stereoselectivity in order to synthesize complex natural compounds like Veratrum alkaloids. Chemistry must meticulously select reactions that can distinguish between analogous functional groups and regulate the production of stereocenters.
Guaranteeing selectivity across various pathways:
A key issue of divergent synthesis is maintaining selectivity among many paths. We employ the same intermediate to generate several products, making it crucial to identify reactions that can selectively functionalize specific regions of the molecule without affecting other parts.
Selective oxidation processes can introduce hydroxyl groups at specific places on the steroidal framework. We must meticulously regulate these reactions to prevent over-oxidation or oxidation of undesired locations.
Synthesis of Complex Alkaloids: Stereochemical Regulation:
Controlling stereochemistry is an important part of making Veratrum alkaloids because these chemicals have many stereocenters that are needed for them to work as drugs. A single error in stereochemical control may yield an inert or less effective molecule.
Chemists frequently employ asymmetric catalysis or chiral auxiliaries to achieve fine control over stereochemistry. These approaches provide the targeted synthesis of the necessary stereoisomers, ensuring that the final product has the correct configuration at each stereocenter. Syntheses of (–)-germine (2) and (–)-protoverine (3).
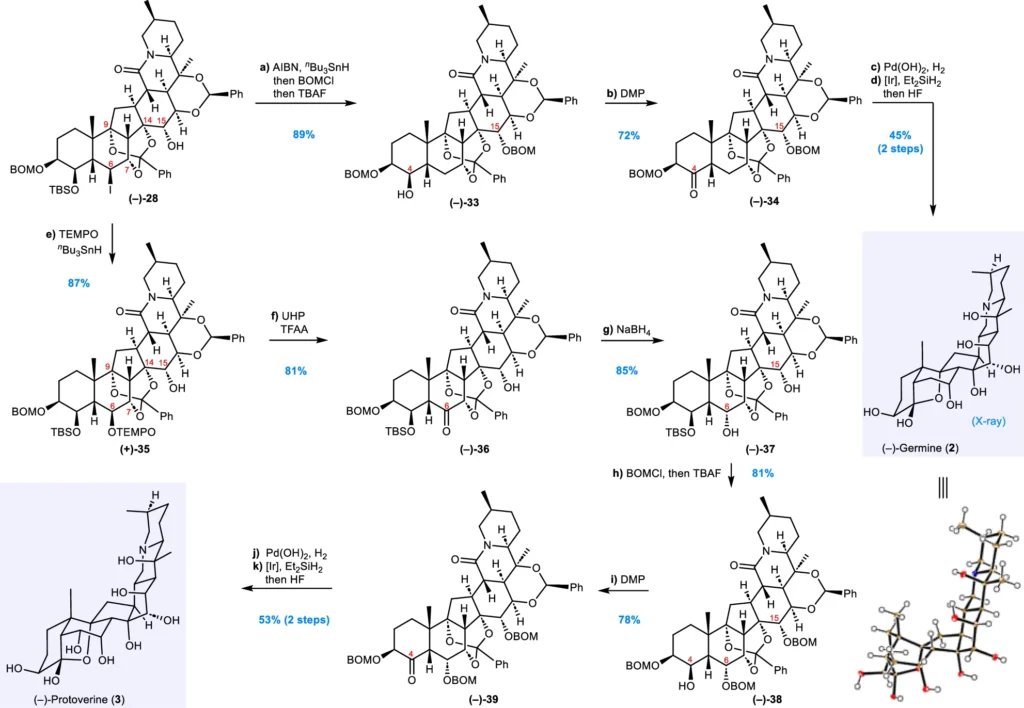
Comprehensive Synthesis of Exemplary Veratrum Alkaloids:
The entire synthesis of intricate natural compounds such as Veratrum alkaloids is a formidable endeavor, necessitating the meticulous coordination of several processes. Recent advancements in divergent synthesis have enabled more efficient access to these alkaloids than previously conceivable.
Synthesis of Veratramine:
Veratramine is one of the major alkaloids in the Veratrum family, and its complete synthesis has been a long-standing objective for synthetic chemists. Scientists have synthesized veratramine using a divergent method, starting from a common intermediate and selectively functionalizing it to create the final product.
The synthesis of veratramine necessitates a number of important processes, including the construction of the steroidal core through cyclization reactions and the insertion of functional groups through selective oxidation and amination reactions. Chemists have streamlined the synthesis of veratramine by employing a common intermediate, thereby reducing the total number of required steps.
Synthesis of Cyclopamine:
Cyclopamine is another essential Veratrum alkaloid, renowned for its ability to block the Hedgehog signaling pathway. The synthesis of cyclopamine is notably difficult owing to its intricate polycyclic architecture and the existence of several stereocenters.
Chemists have built the core structure of cyclopamine using a divergent synthesis strategy. This was done through a series of selective cyclization and functionalization reactions. Asymmetric catalysis has been critical in achieving the appropriate stereochemistry at each stereocenter, facilitating the synthesis of biologically active cyclopamine.
Obstacles to Synthesizing Complex Veratrum Alkaloids:
Despite the gains in divergent synthesis, major problems remain in the synthesis of complex Veratrum alkaloids. These obstacles stem from the high degree of stereochemical complexity, the difficulty of creating a stiff polycyclic framework, and the need for selective functionalization.
Retrosynthetic Challenges:
One of the key obstacles in the synthesis of Veratrum alkaloids is retrosynthetic analysis, which is the act of breaking down a complex compound into simpler starting elements. Because Veratrum alkaloids have a lot of stereocenters and complicated functionalization, retrosynthesis is very hard because chemists have to come up with ways to make the molecule simpler while keeping important structural features.
Sometimes, chemists find it beneficial to focus on synthesizing a common intermediate that they can selectively modify to create the final product. This method facilitates enhanced flexibility in the retrosynthetic pathway, simplifying the management of the target molecule’s complexity.
Addressing stereochemical complexity:
A significant challenge lies in regulating the stereochemistry of Veratrum alkaloids. These molecules possess several stereocenters, and the stereochemical configuration at each center is essential for biological activity. To achieve precise control over stereochemistry, selective reactions, such as asymmetric catalysis or chiral auxiliaries, must be implemented.
Biomimetic strategies have been used by scientists in some cases to copy the biosynthetic pathways that plants use to make Veratrum alkaloids. These pathways frequently entail enzyme-catalyzed reactions that provide significant stereochemical control, offering a viable solution to synthetic chemistry’s stereochemical dilemmas.
Comparison of Divergent Synthesis and Convergent Synthesis:
Scientists must choose between two principal synthetic methodologies when synthesizing intricate natural compounds such as Veratrum alkaloids: divergent synthesis and convergent synthesis.
Divergent Synthesis:
Divergent synthesis starts with a shared intermediate and diverges to produce a variety of distinct products. This method is especially advantageous for targeting a family of related compounds, as it enables chemists to obtain various alkaloids from a common precursor.
Convergent Synthesis:
Convergent synthesis entails the independent construction of distinct molecular fragments, which are subsequently amalgamated in the final stages. Chemists frequently employ this method for the synthesis of large, intricate structures, allowing them to focus on building individual components of the molecule before assembling them into the final result.
Principal methodological distinctions:
The fundamental distinction between divergent and convergent synthesis resides in the sequence of molecular assembly. In divergent synthesis, chemists initiate with a shared intermediate and subsequently generate various products. In convergent synthesis, chemists construct distinct pieces and subsequently amalgamate them in the final stages.
Advantages of Divergent Synthesis for Complex Natural Products:
Divergent synthesis has several significant advantages for complicated natural compounds, such as Veratrum alkaloids. Chemists can synthesize several alkaloids from the same intermediate, thereby enhancing process efficiency through reduced stages. Divergent synthesis gives you more freedom to change functional groups and control stereochemistry, which makes it easier to target different alkaloid family members.
Applications of Veratrum Alkaloid Synthesis:
The ability to produce Veratrum alkaloids has profound implications for both medicine and biology. Research has proven that these compounds possess a wide spectrum of biological functions, making them attractive leads for medication development.
Pharmaceutical Applications:
Veratrum alkaloids have shown potential as therapies for a range of illnesses, including hypertension, inflammation, and cancer. Cyclopamine, in particular, has been studied for its ability to suppress the Hedgehog signaling system, which is linked to the development of certain types of cancer.
In addition to their direct therapeutic benefits, Veratrum alkaloids can also serve as lead chemicals for the creation of novel medicines. By synthesizing these alkaloids in the laboratory, chemists can change their structures to improve their potency, selectivity, and safety.
The potential for bioactive compound development:
The production of Veratrum alkaloids also paves the way for the discovery of novel bioactive molecules. Scientists can study the structure-activity relationships of these compounds by using divergent synthesis to make many alkaloids from a common intermediate. This lets them find out which structural properties are important for biological activity.
Future Directions in Divergent Synthesis:
The future of divergent synthesis depends on the continued development of new catalytic approaches and automation integration. Advances in machine learning and computational chemistry offer the potential to revolutionize the area, allowing for even more efficient and selective syntheses.
Emerging Techniques and Technologies:
Several developing methodologies and technologies are likely to have a role in the future of divergent synthesis. For instance, flow chemistry, which employs continuous flow reactions instead of batch mode, presents the potential for enhanced efficiency and scalability in the synthesis of complex natural compounds.
Another developing method that has shown promise for the synthesis of complex compounds is photoredox catalysis, which uses light to drive chemical processes. By employing light to stimulate certain reactions, chemists can gain improved selectivity and efficiency in their syntheses.
Prospects for Automation in Complex Alkaloid Synthesis:
Automation in organic synthesis is on the rise, potentially leading to the manufacturing of complex alkaloids like Veratrum alkaloids with minimal human interaction. Advances in machine learning and robotics are already starting to have an impact on the discipline, allowing chemists to automate common operations such as reaction optimization and product isolation.
In the future, it may be able to fully automate the synthesis of complex natural products, allowing for the rapid and efficient manufacture of bioactive substances. This will not only make these compounds more accessible for study and development, but also open the door to new discoveries in the field of medicinal chemistry.
Conclusion:
Over the past few decades, the synthesis of complex Veratrum alkaloids has dramatically evolved, largely due to the discovery of divergent synthesis techniques. By starting with a common intermediate, chemists can now access various alkaloids more effectively than ever before. This technique has not only expedited the production of these molecules, but has also opened up new opportunities for the development of bioactive substances.
As new catalytic methods and technology continue to emerge, the future of Veratrum alkaloid synthesis looks bright. Automation and machine learning combine to accelerate the field, enabling the creation of complex natural products and their investigation in medicine and biology.
Frequently Asked Questions:
1). What are veratrum alkaloids?
Veratrum alkaloids are intricate natural compounds obtained from plants belonging to the Veratrum genus. Biological activity and structural intricacy, characterized by a hard steroidal core and numerous stereocenters, distinguish them.
2). What is the significance of veratrum alkaloids?
These alkaloids have demonstrated efficacy as therapies for various illnesses, including hypertension, pain, and cancer. Cyclopamine, a Veratrum alkaloid, is significant for its capacity to disrupt the Hedgehog signaling system, a crucial focus in cancer research.
3). What constitutes divergent synthesis?
Divergent synthesis is a methodology in which a shared intermediate facilitates the creation of a variety of unique products. This method is especially effective for synthesizing groups of related natural compounds, such as Veratrum alkaloids.
4). What are the difficulties associated with synthesising Veratrum alkaloids?
The principal challenges include regulating stereochemistry, assembling the polycyclic framework, and selecting and incorporating functional groups. These molecules possess several stereocenters and are extensively functionalized, rendering their synthesis with high precision challenging.
5). What is the potential impact of automation on Veratrum alkaloids synthesis?
Advancements in automation and machine learning have the potential to transform the synthesis of intricate natural products. In the future, the complete automation of Veratrum alkaloid synthesis may render these molecules more accessible for study and pharmaceutical development.
For more chemistry blogs, visit chemistry Master